Essential Ion and Compound Balance and Homeostasis - Anatomy & Physiology
Sodium
It is very important to regulate the bodies sodium level. If it falls too low then water and ECF volumes also fall and sodium dependant transporters are also disturbed. If it rises too far the transport systems are still disturbed but body water and ECF volume increases. Sodium is therefore maintained within near constant parameters. With the amount being excreted/lost corresponding to that ingested. Salt intake is not really regulated and thus it falls to the kidneys to maintain salt levels via excretion.
Regulation
The total body content of sodium is regulated rather than the actual plasma concentration. It would be impossible to regulate actual plasma concentration for 3 reasons:
- When sodium is reabsorbed water follows it so the volume of the plasma is altered rather than the concentration of sodium changing
- Angiotensin 2 and Aldosterone affect sodium but they also affect ECF volume so only quantity affected not concentration
- ADH and thirst response also work together to dilute the ECF if concentrations of sodium are high so although there is more NaCl the actual concentration is not really changed.
The kidneys are the most important regulatory organs of sodium in the body. They adjust their excretion to match the amount taken in through the digestive tract taking into account the amount lost through sweat. The handling of sodium by the kidneys is also essential to allow the reabsorption of many other important nutrients which would otherwise be lost in the urine. These nutrients include glucose, amino acids, chloride, bicarbonate and phosphate. It is also exchanged for the likes of potassium and hydrogen ions to aid in their secretion. As a result sodium transport accounts for over 80% of the energy metabolism in the kidneys
Salt Hunger
The diet of a herbivore contains little sodium and as such an important aspect of their sodium homeostasis is the physiological salt appetite where the animal actually craves salt if it is deficient. This is especially apparent in sheep. By contrast carnivores have a very poorly developed physiological salt appetite probably because their salt intake tends to outstrip their requirement.
Potassium
Importance of Regulation
Decreased Extracellular Potassium
If the concentration of potassium in the ECF is reduced then the plasma membranes hyperpolarize resulting in decreased firing of action potentials. This causes skeletal muscle weakness and cardiac abnormalities.
Increased Extracellular Potassium
In this state the membrane is depolarised and is inappropriately triggered by action potentials. This can make the membrane insensitive to further stimulation causing cardiac abnormalities.
Sources
- Potassium is absorbed via passive diffusion from the small intestine
- Also via active transport from the colon
- Affected by Aldosterone
- Highly efficient
- It's recovered from cellular breakdown
- Haemolysis
- Tissue damage
Methods of Control
The K+ in the ECF only represents a very small amount of the total K+ in the body however its concentration is maintained within very strict parameters. The homeostasis of K+ is managed by three routes:
Cellular translocation
- Potassium is moved either into or out of the cells
- Acute response
- Main method of control
Renal excretion
- Makes up 90% of the chronic response
- Takes 4-6 hours to respond
- Allows fine control
- Influenced by aldosterone
GI excretion
- Makes up the other 10% of the chronic response
- Also influenced by aldosterone
- Most important in renal failure
Cellular Translocation
- Vital for rapid control of potassium loads
- Helps control plasma concentration
- Moves potassium into the cell
- Stores potassium in skeletal muscle and liver
- Balances ECF and ICF
- Controlled by insulin and beta2 adrenoreceptors
- Increases the activity of Na+ / K+ ATPases causing sodium efflux and potassium influx
Renal Control
- Potassium ions are reabsorbed and secreted at different points along the nephron
- Active reabsorption of potassium occurs along the proximal tubule (70%) and along the ascending limb of the Loop of Henle (10-20%)
- This results in there only being 10% of the original amount left in the distal tubule
- However net reabsorption / secretion of potassium occurs in the distal tubule and first part of collecting duct
- Depends on bodies need
- Under the influence of aldosterone
- This is where the amount of potassium excreted is determined
- Reabsorption occurs in the final part of the collecting duct
Potassium and Aldosterone
- Aldosterone is the most important regulator of potassium
- It causes increased secretion of potassium
- Increased potassium directly stimulates aldosterone secretion
- Increases the activity and number of Na+ / K+ ATPase in basolateral membranes of the principal cells in the collecting duct and distal tubule
- Potassium moves into the cells and is then excreted down an electro-chemical gradient
Factors Influencing Potassium Excretion
Sodium
- High sodium = increased potassium excretion
- More sodium into cells
- Increased Na+ / K+ ATPase
- Pumps sodium into peritubular renal interstitium
- The resulting increased cellular uptake of potassium results in it moving down the electrochemical gradient into the nephron
Potassium
- High potassium = increased potassium excretion
- Triggers aldosterone
Acid-Base
- Potassium is exchanged for H+
- Hypokalaemia = potassium to ECF and hydrogen to ICF
- Results in alkalosis
Acidosis
- Potassium moves from ICF to ECF
- pH change of 0.1 leads to potassium change by 0.6mmol/l
- (rough guide)
Alkalosis
- Opposite effect
- Potassium moves from ECF to ICF
Acid / Base
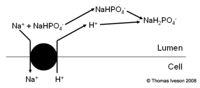
It is essential that 50-100meq of acid is excreted by the kidneys every day. This is achieved by secretion of H+ in two regions of the nephron, the proximal tubule and the collecting ducts, and is essential for maintaining the acid base ratio, within the body, at the correct levels. If there is a net gain or loss of H+ within the body then the kidneys will compensate for it. The H+ ions cannot be secreted as free ions, however virtually all filtered HCO3- must be reabsorbed. The result is that the H+ ions bind to other filtered buffers which are not fully reabsorbed such as ammonia or phosphate. Extracellular pH is the main physiological regulator affecting how much acid is secreted. In pathological states circulating volume, aldosterone and plasma potassium affect it.
The Role of the Kidneys in Acid Base
The kidneys work with the respiratory system to regulate H+. Where as the respiratory systems quickly compensates for a problem it is left to the kidneys to actually remove the problem and restore a proper balance. They do this by altering the plasma concentration of HCO3-.
Alkalosis
In a situation of alkalosis the kidneys allow more HCO3- to be excreted. This results in an increase of un-buffered H+ and thus returns the pH towards normal.
Acidosis
In a situation of increased H+ levels the body is said to be in a state of acidosis and the kidneys stop excreting HCO3- and the tubular cells produce more bicarbonate. This results in more H+ being buffered and the pH increases back to normal
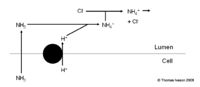
Buffers of H+ in Urine
- Weak acids are filtered and act as buffers
- Ability depends upon pKa+ and concentration
- Once all the bicarbonate has been reabsorbed the secreted H+ combine with these instead.
- The H+ ion is then excreted
The Role of Ammonium in the Proximal Tubule
- The body is able to excrete H+ as ammonium NH4+. This is very useful as:
- It adds flexibility to renal acid base regulation and can help regulate NH4+
- It is ionised, fat insoluble and trapped therefore is excreted
- It is easily replaced so is quite a good method
- Under physiological control
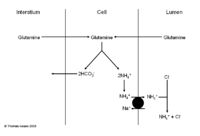
The Role of Ammonium in the Collecting Ducts
- Very different mechanism
- No bicarbonate produced
- See diagram
The Effect of The Buffers on Body pH
- When H+ is binding to buffers other than bicarbonate it is excreted. This means that the reabsorption of bicarbonate represents a net gain of bicarbonate not just a replacement of what was filtered. This results in an increase in plasma pH.
- Bicarbonate is also produced as part of the secretion of ammonium in the proximal tubule
Regulation
- H+ is important for both HCO3- reabsorption and generation of new HCO3-
- However the rate of secretion must be carefully regulated
- There must be enough H+ secreted to reabsorb all the filtered HCO3-
Calcium
Calcium Homeostasis has three aspects:
- Renal reabsorption - dealt with here
- Intestinal absorption
- Exchange processes from the bones
Urea
Urea and Foregut Fermenters
If species such as cows are fed a low nitrogen diet then a smaller portion of the filtered urea is excreted. Indicating that it is being transported to the fore stomach for processing into microbial protein. This allows the animal to survive longer on low protein diets
Protein in Feed and Urine Concentration
- If feed is high in protein then lots of urea is produced and the urine is more concentrated
- If feed is low in protein then less urea is produced and the urine is less concentrated
Revision
Use the flash card revision resource for this section to test yourself.