Osmosis and Filtration - Anatomy & Physiology
Introduction
Osmosis is the passive movement of water across a semi permeable membrane. It occurs in the opposite direction to diffusion of ions. Water moves from a region of low solute concentration and therefore high water concentration to a region of high solute concentration and low water concentration.
Pressure and Water Movement
If you have two bodies of water, one pure and one with a solute dissolved in it, separated by a membrane permeable to water but not the solute then water molecules from the pure water move to the side with the solute. This movement of water causes a fluid pressure difference at the entrance and exits of the holes in the membrane. At the side with the solute the fluid pressure is lower than at the side without however this pressure difference is only at the exit of the hole and not in the solution as a whole. This pressure difference is the actual cause of the movement of water.
However if you increase the pressure, on one side of the membrane, of the fluid as a whole you force more water through the membrane. If enough pressure is applied to the side with the solute then it can overcome the pressure differences across the pores and reverse them causing water to flow against its concentration gradient. This is filtration. The pressure of a fluid is termed the hydrostatic pressure. The point at which the hydrostatic pressure prevents the natural movement of water is called the osmotic pressure. As the concentration of the solution becomes higher the greater the pressure required to overcome the natural movement of water therefore the greater the osmotic pressure.
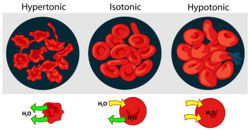
Water therefore moves from areas of lower osmotic pressure to areas of higher osmotic pressure however if you increase the pressure on the side with the higher osmotic pressure you can still stop the net movement of water.
If two solutions are of the same osmotic pressure they are termed isosmotic, a solution with a higher osmotic pressure is termed hyperosmotic and one with lower is termed hyposmotic.
Units of Pressure
Pressure is force per unit area. Therefore its si unit the Pascal is N/m2. However it is common in physiology to use mmHg which is the measure of pressure at the bottom of a column of mercury of that height.