Feline infectious disease
Chlamydia felis (formerly Chlamydophila felis)
Chlamydia felis (formerly Chlamydophila felis and C. psittaci var. felis) predominantly causes conjunctivitis in affected cats but may be involved in upper respiratory tract disease. Infection can become endemic in some cat colonies and recurrent signs are common. An antigen ELISA test and PCR are available for diagnosis. PCR is more sensitive. Conjunctival, nasal or pharyngeal swabs (plain) may be submitted but the organism is reportedly shed predominantly in conjunctival secretions. Samples are collected by reflecting the eyelid and rolling the swab vigorously across the conjunctiva to harvest cells. If necessary, the swab can be moistened with sterile saline prior to sampling. If submission is delayed, then samples may be stored at 2-8°C for up to 48 hours.
Feline leukaemia virus
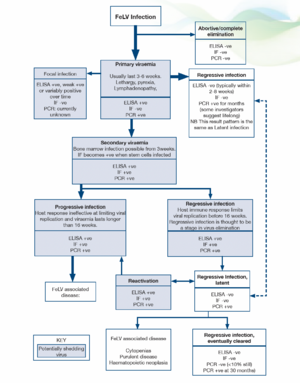
General. The prevalence of FeLV is not clear, but has probably fallen to <1% (Gruffyd Jones 2006). With the introduction of PCR testing our understanding of the pathogenesis and outcomes of infection has been challenged. More recent proposals have suggested the following stages in infection and FeLV status categories (Greene 2012):
Abortive/complete elimination. Virus replicates in local oropharyngeal lymphoid tissue but systemic spread is prevented by humoral and cell-mediated responses. Exposure to infection is indicated by the presence of antibodies but cats are negative for antigen and proviral DNA. It is unclear how often this occurs in natural infection but it is likely to be very uncommon. Studies using newer, sensitive PCR techniques indicate that proviral DNA may be detected at a later date in many patients previously believed to have eliminated the virus. However, the clinical significance of persistence of proviral DNA is unclear since the life expectancy is the same as for cats which have never been infected.
Regressive infection (transient viraemia). Infected cats become viraemic (primary viraemia) but an effective antibody response is mounted and virus replication/viraemia is terminated before or shortly after bone marrow infection (around 3 weeks post infection). In most cats the primary viraemia lasts 3-6 weeks (maximum 16 weeks) during which time, FeLV p27 antigen (ELISA) is detected in the plasma and cats are infectious. Previously, it was thought that termination of the viraemia was accompanied by rapid, total elimination of the virus from all cells (based on negative ELISA results within 2-8 weeks). However, recent studies suggest that FeLV becomes integrated into the cat’s genome and thus can be detected by qPCR for months after the initial viraemia. It has been proposed that integration of proviral DNA is essential for solid protective immunity and some investigators propose that small quantities of proviral DNA may actually persist for life.
The clinical significance of an antigen negative/proviral DNA positive pattern is unclear but cats with this outcome of infection have effective immunity, are likely protected against new exposure and have a low risk of developing FeLV associated diseases. They are unlikely to shed virus via oronasal secretions but may pose a risk to other cats via blood transfusion.
Some cats do not clear the primary viraemia prior to infection of the bone marrow precursors (secondary viraemia) and in these patients viral antigen is detected within platelets and granulocytes by immunofluorescence testing (IF). Once infection is established in the stem cells the virus cannot be eliminated from the bone marrow by the host immune response and most of these patients go on to develop progressive infection (see below). Progressive infection is more likely when viraemia persists for more than 16 weeks. However, in a small number of cats the immune response does limit systemic replication of the virus shortly after bone marrow infection. Although proviral DNA persists in the stem cells of these cats, it is not translated into proteins and affected individuals do not produce virus, become negative for FeLV antigen (ELISA, IF) and are not infectious to other cats. This is termed latency and may be a permanent state but it appears that the majority of cats lose functional viral material for example, through gene reading errors during cell division by 16 months after infection, with only 10% still affected at 30 months.
Regressive (latent) infections may reactivate if the host immunity wanes for example, during pregnancy, periods of stress or after high doses of glucocorticoids. Reactivation is more likely if the stressful event occurs shortly after the initial viraemia and is considered unlikely to occur after 2 years.
Regressive infections are likely to be a stage in the elimination of the virus. Most regressive infections are not clinically significant (reactivation is uncommon under non-experimental conditions) but clinical signs may occur and include cytopaenias, suppurative inflammatory conditions and haematopoietic neoplasia.
Progressive infection
In progressive infections there is an ineffective immune response allowing extensive viral replication in lymphoid tissues, bone marrow and mucosal/glandular tissue, accompanied by virus shedding. If the viraemia persists unchecked for 16 weeks then cats remain persistently viraemic and infectious and most develop FeLV related diseases, often within 3 years. FeLV DNA provirus is detected in both regressive and progressive infections and these can only be differentiated by repeat antigen tests, which in regressive infections become negative after 2-8 weeks, or occasionally, after some months.
Focal Infections
Focal infections of specific tissues (mammary gland, spleen, lymphoid tissue, small intestine) can lead to low grade or intermittent virus production and affected cats may have weak positive or discordant results (ELISA, IF) or variable results over time. They should be considered a potential source of infection to other cats.
Tests for FeLV
ELISA (Enzyme-linked immunosorbent assay)
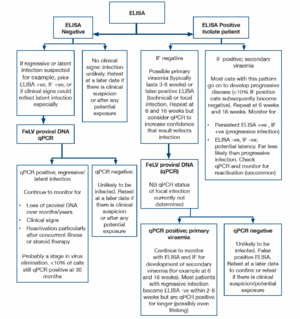
ELISA and rapid immunomigration methodologies are commonly employed in in-clinic tests. They detect free viral protein (p27) in plasma, tears or saliva. Testing serum or plasma is preferred and the use of saliva or tears is not recommended. The specificity of many of these tests is 98-99%, but because of the very low incidence of FeLV, approximately half of the positive tests will be false positives. True positive results could reflect transient or persistent viraemia and further testing (by immunofluorescence) is recommended initially. If immunofluorescence (IF) is initially negative repeat testing 6 and 16 weeks after the initial test is indicated to determine whether the patient has a regressive infection (transient viraemia) or progressive infection. Cats with a persistent ELISA positive, IF negative pattern may have a focal infection.
IF (Immunofluorescence)
IF detects viral protein (p27) in the cytoplasm of leucocytes (predominantly neutrophils) and platelets. Positive results are highly likely to reflect persistent infection, <10% of cats with positive IF result have regressive infections (transient viraemia). IF is a suitable staging test for positive results obtained using ELISA, but is not recommended as an initial screening test because cats with primary viraemia (which may be infectious to other cats) are not detected. False negative IF results may be noted in cats with neutropaenia and/or thrombocytopenia.
FeLV DNA (provirus)
FeLV proviral DNA qPCR allows quantification of FeLV DNA in the blood or bone marrow. High levels are found in ELISA and IF positive cats, low levels are found in cats which are negative using antigen methods. These cats may have latent infections and qPCR is recommended where there is a strong clinical suspicion of FeLV-related disease but negative antigen tests. Cats with regressive and progressive infections are expected to be positive for FeLV proviral DNA thus qPCR will not allow differentiation in ELISA positive patients, particularly in acute infection. In the future, quantification of the viral load in leucocyte subsets may allow differentiation later in the infection, when most cats with virus in the granulocytes have progressive infection while cats with regressive infection appear to possess only a small quantity of proviral DNA in lymphocytes.
As a retrovirus, FeLV mutates naturally and a mutated viral strain may not be detected using specific primers. A negative result does not, therefore, exclude infection.
FeLV infection status
The likely FeLV status of a cat can be determined using the results of ELISA, IF and proviral DNA. However, collection of multiple samples over weeks/months may be required for clarification, particularly for differentiation between regressive infection (transient viraemia) and progressive infection (persistent viraemia). Most cats (>90%) with a positive IF result have progressive infection.
Cats with discordant results (ELISA positive, IF negative) may have a false positive ELISA result, a primary viraemia or focal infection. Isolation of the patient, followed by retesting in 6 weeks and 16 weeks is recommended and provides clarification of the status in many patients. A positive qPCR test would indicate infection and allows confirmation of the infection status (false positive ELISA versus infection). However, a negative qPCR result does not completely exclude infection. Further testing may be required for some individuals.
| Status | ELISA | IF | FeLV DNA |
|---|---|---|---|
| Not infected | - | - | - |
| Abortive/complete elimination | - | - | - |
| Regressive (primary viraemia; no secondary viraemia | + | - | + |
| Regressive (with secondary viraemia)* | + | + | + |
| Regressive; latent | - | - | +** |
| Progressive | + | + | + |
| Focal | +/- | - | Currently unknown |
*Most cats with this pattern have progressive disease
**Positive more likely in bone marrow than peripheral blood
When to test for FeLV
Testing is recommended when:
- Cats present with clinical illness (irrespective of previous test results)
- Cats are to be re-homed (even if there are no cats currently in the household). Cats with negative results should be re-tested after at least 4 weeks. Kittens may be tested at anyage but kittens which have been infected by maternal transmission may not be positive for to months after birth (once the virus starts replicating)
- There has been potential exposure to FeLV. If testing is negative then it should be repeated after at least 4 weeks (ideally also after 12 weeks). In a cat with a single exposure the risk of developing progressive infection averages 3% (Greene 2012)
- Cats with high risk lifestyles or living in households with infected cats should be tested on a regular basis. Introduction of an FeLV shedding cat into a group of naive cats for an extended period increases the risk of naive cats developing progressive infection to 30% (Greene 2012)
- Prior to FeLV vaccination. FeLV vaccination does not affect the test result but samples should not be collected shortly after vaccination since some investigators report the risk of detecting vaccine antigens. The duration of this phenomenon is unclear
- Screening of blood donors, using antigen detection methods and qPCR
Feline immunodeficiency virus
Tests for FIV
The prevalence of FIV is reported as 4-6% in the general cat population and 12-18% in sick cats (Gruffyd Jones 2006). Tests for FIV infection detect antibody production using ELISA, immunofluorescence (IF) or Western Blot methodologies. Positive ELISA results should be confirmed by IF. Most cats have seroconverted by 60 days post infection, some take longer; in addition, antibody production in some individuals may be insufficient for detection, particularly in the terminal phase of infection. In these patients infection may be confirmed by measurement of FIV (proviral) DNA by PCR.
Confirmation of infection in kittens born to infected queens is problematic since kittens under 6 months of age may have maternally derived antibodies. If a positive ELISA result is obtained in a kitten <6 months, repeat testing at 60 day intervals is recommended. Possible sources of infection should be considered and the kitten’s final test should be no less than 60 days after the last potential exposure. Positive test results in cats over 6 months of age are an indication of infection.
FIV (proviral) DNA may be measured by PCR to confirm infection in kittens in which antibody test results may be misleading and repeat ELISA testing causes an unacceptable delay in diagnosis. In addition, the PCR test can be used to monitor proviral loads in infected cats. The test is reliable for the detection of the clade A subtype which is to date, the cause of infection in the UK.
When to test for FIV
The American Association of Feline Practitioners recommendations include testing cats when:
- They present with clinical illness, even if they have tested negative in the past
- They are to be re-homed. Negative cats should be retested in 60 days
- If a positive result is obtained in a young kitten then further testing is recommended at > 6 months (and no less than 60 days after any potential exposure)
- There has been potential exposure. FIV negative cats should be tested at least 60 days after the potential exposure
- There is a high risk lifestyle. Regular testing is recommended for cats with bite/fight wounds and individuals housed with FIV infected cats
Feline infectious peritonitis (FIP)
Feline Coronavirus infection is common, especially when cats are group housed, however, the majority of infected cats do not go on to develop FIP. FIP is an immune mediated disease, associated with viral mutation which allows replication of the virus in macrophages and monocytes. Following infection with Feline Coronavirus (FCoV), most cats shed virus for a variable period of time although the majority have ceased shedding by 9 months PI. In at least 25% of these cases, virus can also be detected in the blood. Over 90% of cats exposed to FCoV seroconvert; the antibody titre eventually decreases and becomes undetectable. Thirteen percent of cats become lifelong carriers and permanently shed virus (faecal). Only 10% of seropositive cats go on to develop FIP. A small proportion of cats appear to be naturally resistant to infection, they do not develop an antibody response and do not shed virus.
Diagnosis
A definitive diagnosis is difficult; the gold standard requires demonstration via immunostaining, of FCoV antigen within tissue or effusion macrophages, along with consistent histopathological changes. A tentative diagnosis may be made based upon the weight of evidence from the following tests:
- Multicat household. The seroprevalence of FCoV in single cat households is reported as 25% but can be up to 100% in multicat households
- Signalment. Most affected cats are aged between 3 months and 2 years, there is a second peak at >10 years
- History. The clinical signs are often noted following a stressful event such as re-homing or surgery
- Clinical signs. Anterior uveitis, pyrexia, lethargy, anorexia, weight loss, palpable mesenteric lymph nodes, ascites (25% of these also have pleural effusion), neurological signs (seizures, nystagmus, hyperaesthesia)
- Biochemistry. Hyperglobulinaemia, polyclonal gammopathy and A:G <0.7 (<0.40 highly likely, >0.8 FIP unlikely). Raised globulins are present in more than 50% of cats with wet FIP and 75% of cats with the dry form. Other profile changes include normocytic normochromic anaemia, neutrophilia, lymphopaenia and proteinuria if there is renal involvement
- Hyperbilirubinaemia. In the absence of pre-hepatic, intra- hepatic or post-hepatic causes. Hyperbilirubinaemia of sepsis is thought to reflect impaired transport of bilirubin across the canalicular membrane, due to high levels of TNF alpha
- Abdominal effusion. Total protein >35g/l and A:G ratio <0.7 (<0.40 highly likely, >0.8 FIP unlikely)
- FCoV antibodies (IF). Serology can be performed on serum or effusion. Cats from a multicat household or rescue centre within the previous 6-12 months are likely to have antibodies to FCoV. Approximately 9 out of 10 of these cats will not develop FIP
- FCoV antibody titre. In dry FIP the titre is often >1280. Wet FIP titres vary from 0 to >2560.
- APP. Serum or effusion alpha 1-acid glycoprotein >1.5g/l
- PCR. Detects viral genetic material in effusions and is useful in seronegative cats with suspected wet FIP. A positive result significantly increase the index of suspicion. A negative result does not exclude the diagnosis
Serological testing in other circumstances
Testing when there is suspected exposure of a healthy cat
FCoV is highly infectious and most in-contact cats will be seropositive. Only 10% of infected cats will develop FIP. The following may be useful:
A seronegative cat is unlikely to develop FIP and is not shedding virus. However, a small number of cats with wet FIP have a titre of 0. It is probably safe to get another cat, but the new cat should also be seronegative.
There is a one in ten chance of a seropositive cat developing FIP and a one in three chance that the cat is shedding virus. It is probably unwise to bring in a new cat at this time. Retest at 3-6 month intervals. The titres eventually decline in most unaffected cats.
Screening before mating.
A seronegative cat is not excreting virus and can be mated to another seronegative cat. A seropositive cat should be mated to a cat which is also seropositive.
Screening a cattery
Since FCoV is highly infectious, sampling 3-4 individuals at random gives an overview of the status. Cats may eventually become seronegative if they are housed in small groups (<3) or the total number of cats is relatively small (<10). Test every 6-12 months to determine if the proportion of seronegative cats is increasing.
Screening a cat for introduction into a new household
Seronegative cats may be introduced. Seropositive cats may be shedding virus.
Haemoplasmosis (Feline infectious anaemia)
There are three species of haemotrophic feline Mycoplasma species, Candidatus Mycoplasma haemominutum (the small form), Mycoplasma haemofelis (the large form) and Candidatus Mycoplasma turicensis. Infection with C. Mycoplasma haemominutum causes mild anaemia and more severe disease in immunocompromised patients. The other two species cause severe haemolytic anaemia. In some cases the organisms are identified in a blood film made from blood which does not contain an anti-coagulant. A PCR test is available for all three species and is much more sensitive. Post treatment samples can be submitted to determine whether the infection has been cleared successfully. Since the PCR test is quantitative, it may be used during treatment to monitor the therapeutic response. PCR screening of blood donors is highly recommended.
Respiratory viruses (FRV, FCV)
Feline rhinotracheitis virus (FRV; Herpesvirus) and Calicivirus (FCV) are significant causes of respiratory disease in cats. Infection with FRV usually results in a carrier state. During latent periods the virus is not shed and cannot be detected, but shedding often occurs approximately one week after a stressful event and may persist for 1-2 weeks. During shedding the cat is infectious. A carrier state is also a common sequel to infection with FCV but shedding is usually continuous. The infection may eventually be cleared but some cats are long term carriers. In experimental studies, 50% of cats were still shedding virus at 75 days post infection.
Diagnosis
Oropharyngeal swabs (ideally within the first week of illness) in viral transport medium for viral isolation or dry swabs (can be soaked in sterile saline prior to sampling) for testing vio PCR.
Toxoplasmosis
Toxoplasmosis is caused by the intracellular coccidian parasite Toxoplasma gondii, cats are the definitive host. Infection usually results from the ingestion of bradyzoites in tissues of vertebrate intermediate hosts.
The majority of infected cats do not show clinical signs. Transient small bowel diarrhoea may accompany the gut epithelial stage of primary infection. Systemic signs may develop during tachyzoite replication in extra-intestinal tissues and will reflect organ involvement. Hepatic, pulmonary, CNS and pancreatic involvement are common. Risk factors for clinical disease include concurrent retroviral infection, FIP or the administration of immunosuppressive drugs including cyclosporine A.
Clinical signs in acute disseminated infection include anorexia, lethargy, pyrexia or hypothermia, dyspnoea, abdominal effusion, diffuse or multifocal CNS signs, uveitis, jaundice. On routine profiles there may be non-regenerative anaemia, neutrophilia, lymphocytosis, monocytosis, hypoproteinaemia, raised liver enzymes, hyperbilirubinaemia and proteinuria. Serology is a useful component of the investigation, but there is some controversy over interpretation, especially regarding IgM titres when used in isolation for diagnosis. A tentative ante mortem diagnosis may be based upon appropriate clinical signs, exclusion of other differentials, supportive IgG and IgM titres and a positive response to anti-Toxoplasma drug therapy.
Diagnosis
Antibodies (IgG and IgM) to Toxoplasma gondii are identified by IF.
IgG titres
A negative titre (<50) is not consistent with active infection although the cat may have been exposed in the last 3 weeks and seroconversion has not occurred.
A positive titre (50 or >50) indicates exposure, but need not reflect active disease. Some cats do not develop an IgG titre until 4-6 weeks after infection and then maximal titres are reached within a further 2-3 weeks. There is some evidence that higher titres (>400) are associated with active infection but other investigators suggest that serology should be used only as a screening tool to rule out exposure. Very high IgG titres (>800) may be noted where rheumatoid factor (RF) is present. RF may cause false positive or negative IgM results.
Paired serum samples taken 2-4 weeks apart can also help in the interpretation of positive titres. An increase of ≥4 fold indicates recent or active infection (true positive).
A <4 fold increase in titre does not support recent or active infection (true negative), unless the maximal titre was achieved before taking the first sample or the titre failed to rise, as with recrudescent disease (false negative).
High titres can persist for many years due to latent infection (bradyzoite tissue cysts).
IgM titres
IgM antibodies are commonly identified in the serum of clinically ill cats and not healthy cats, but titres should still be interpreted with care. Titres of 20 and above are likely to be associated with active infection (when accompanied by an appropriate IgG titre), but titres may persist with chronic infection in some healthy cats, cats with concurrent FIV or those receiving glucocorticoid therapy. The presence of this antibody class does not accurately predict the period of oocyst shedding. Positive IgM titres with negative IgG titres should be interpreted with caution since there may be some cross-reactivity with other parasites. Ideally, a second sample should be tested in 2-3 weeks to check for a rising IgG titre. The presence of rheumatoid factor (Toxoplasma IgG titre usually >800) may cause false negative and positive IgM results. A definitive diagnosis of Toxoplasmosis requires detection of tachyzoites in body cavity effusions, BAL fluids, CSF, tissue aspirates or biopsy material via cytology, histology or immunohistochemistry. The sensitivity is low but specificity is high.
Screening a cat whose owner is pregnant
Negative IgG titre
(<50) No evidence of exposure (although the cat could have been exposed in the last 3 weeks and not yet seroconverted). The patient is at risk of infection from hunting and eating undercooked meats. Indoor cats fed on a tinned diet are unlikely to become infected but faeces should be removed from the litter tray within 24 hours, ideally by a non-pregnant individual. Unsporulated oocysts are not infective but sporulation occurs within 1-5 days. If litter trays are cleaned and disinfected daily any risks from cleaning them should be minimised.
Positive IgG titre
(50 or >50) The cat has been exposed. In general, oocysts are shed for only 1-2 weeks post infection. Cats are unlikely to be shedding once an IgG titre has developed, however, some cats may shed low numbers of oocysts if re-challenged or treated with immunosuppressive doses of prednisolone. Potential exposure to oocysts should still be minimised (as above). Most human infection is acquired following ingestion of tissue cysts in undercooked meat or oocysts from the environment (contaminated vegetables, gardening without wearing gloves or inadequate hand washing etc).