Hypoadrenocorticism
Jump to navigation
Jump to search
Hypoadrenocorticism (Addisons disease)
Pathogenesis: Type IV hypersensitivity
Primary Hypoadrenocorticism
Multiple aetiologies, including:
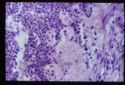
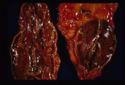
- Adrenal atrophy: Adrenals are grossly small and difficult to find. They are dark brown on cut section. On histology there will be an infiltrate of lymphocytes, plasma cells and macrophages. Thought to be auto-immune, also see increased incidence of other immune mediated diseases E.g. IMHA.
- Adrenal necrosis: May be:
- Infectious in aetiology E.g Salmonellosis in horses. Necrosis may also occur due to:
- Myoarteritis in uraemic animals resulting in ischaemia of the adrenal gland.
- Another possible type of necrosis is idiopathic.
All are seen grossly as areas of red haemorrhage and yellow necrotic foci.
- Bilateral adrenalectomy/Mititane therapy: Mitotane selectively destroys the zonas fasciculata and reticularis while sparing the essential zona glomerulosa. Used in the treatment of Cushings disease. Iatrogenic Addisons disease is a common sequale of treatment.
Secondary Hypoadrenocortisism
Deficient pituitary secretion of ACTH. Often iatrogenic due to withdrawal of glucocorticoid treatment. Prolonged high dose treatment induce adrenal atrophy due to the effect of negative feedback on the pituitary. The withdrawal of drug must be gradual to allow to adrenal gland to return to function over a period of time. Usually little effect on mineralocorticoids as ACTH has little trophic effect on their production.
Pathophysiology
- Aldosterone deficiency: Aldosterone normally acts to aid sodium reabsorption and potassium excretion in the kidney. With deficient aldosterone the animal will have:
- Hyponatraemia.
- Hypochloraemia.
- Hyperkalaemia.
- Glucocorticoid deficiency: Cortisol is the stress hormone which acts to increase blood glucose in stressful situations (amongst other functions). Deficiency leads to the following:
- Hypoglycaemia.
- Increased circulating lymphocytes and eosinphils due to removal of the immunosuppressive glucocorticoids.
- Increased skin pigmentation; Low levels of glucocorticoids allow increased ACTH production as negative feedback on the pituitary is removed/decreased. As ACTH is released, so is MSH therefore a change in skin pigmentation indicates endocrine disease.
Clinical signs:
- Acute necrosis will present as an acute syndrome with hypovolaemic shock, vomiting and collapse.
- Chronic damage to the adrenal gland will result in dehydration, diarrhoea, anorexia and weakness.
Diagnosis:
- Haematology:
- Haemoconcentration in acute crisis; due to rapid dehydration.
- Non-regenerative anaemia in chronic form; glucocorticoid deficiency decreases erythropoeisis.
- Electrolyte imbalance: as above.
- Pre-renal azotaemia: elevated urea and creatinine.
- ACTH stimulation test: Positive test is low initial cortisol with no response to ACTH.
- ECG change: Due to hyperkalaemia. In severe cases may see P-wave absence and sino-atrial standstill.
Treatment:
- Acute crisis: Rapid i/v saline and i/v glucocorticoids.
- Chronic form: Fludrocortisone acteta to replace mineralocorticoids. Add table salt to food and give glucocorticoids in times of stress E.g. transport.