Difference between revisions of "Nasal exudates and masses"
Fiorecastro (talk | contribs) (Created page with "Clinical signs associated with nasal disease may include sneezing, nasal or occular discharge, epistaxis, pawing at the nose and facial deformity. Epistaxis may also be seen i...") |
Fiorecastro (talk | contribs) |
||
| Line 1: | Line 1: | ||
| − | Clinical signs associated with nasal disease may include sneezing, nasal or occular discharge, epistaxis, pawing at the nose and facial deformity. Epistaxis may also be seen in primary or secondary coagulopathies and hyperviscosity syndromes. | + | [[File:NationWide Logo.jpeg|right|link=https://www.nwlabs.co.uk/|alt=NationWide Logo|240x240px|frameless]]Clinical signs associated with nasal disease may include sneezing, nasal or occular discharge, epistaxis, pawing at the nose and facial deformity. Epistaxis may also be seen in |
| + | |||
| + | primary or secondary coagulopathies and hyperviscosity syndromes. | ||
Rhinoscopy and cytological evaluation are simple non-invasive procedures which may lead to a definitive diagnosis. Cytological findings must be evaluated with historical, physical, rhinoscopic, radiographic and clinical laboratory findings to maximise the likelihood of achieving a diagnosis. | Rhinoscopy and cytological evaluation are simple non-invasive procedures which may lead to a definitive diagnosis. Cytological findings must be evaluated with historical, physical, rhinoscopic, radiographic and clinical laboratory findings to maximise the likelihood of achieving a diagnosis. | ||
| Line 6: | Line 8: | ||
Cytological specimens are collected from the nasal cavity by flushing or aspiration techniques which should be performed after radiography and rhinoscopy so that radiographic detail and visualisation of the cavity is not altered by haemorrhage and manipulation of nasal tissue. | Cytological specimens are collected from the nasal cavity by flushing or aspiration techniques which should be performed after radiography and rhinoscopy so that radiographic detail and visualisation of the cavity is not altered by haemorrhage and manipulation of nasal tissue. | ||
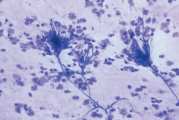
| − | [[File:NWL Mycotic rhinitis showing fungal hyphae and fruiting bodies.jpg | + | [[File:NWL Mycotic rhinitis showing fungal hyphae and fruiting bodies.jpg|thumb|Mycotic rhinitis showing fungal hyphae and fruiting bodies ©NationWide Laboratories 2017|alt=|center]]General anaesthesia is required, with an endotracheal tube in place and the cuff inflated to prevent inhalation of blood and fluids during sample collection. Care must be taken not to penetrate the cribriform plate with the instruments used for collecting samples. Some haemorrhage will occur during manipulation of the nasal cavity, but this should not be a complicating factor unless the animal has a coagulopathy, in which case these procedures are contraindicated. |
| + | |||
| + | == Nasal flushing technique == | ||
| + | |||
| + | * Pack the caudal pharynx with absorbent material | ||
| + | * Estimate the depth of the nasal cavity by measuring the distance between the external nares and the medial canthus of the eye. Pass a soft rubber catheter either caudally through the external nares or rostrally from the nasopharynx. Attach a syringe containing approximately 20ml of sterile normal saline, then using alternate positive and negative pressure repeatedly flush the nasal cavity before retrieving fluid and harvest of material. A smaller volume is used in patients <5kg, for example 10ml | ||
| + | * Collect dislodged particulate matter on a gauze sponge caudal to the soft palate or at the nares, depending on the direction of flush. Collect aqueous material by aspiration | ||
| + | * Transfer the fluid into two plain tubes and one EDTA tube. Add 2 drops of formalin to one plain tube and label accordingly. Make a direct air-dried smear of any large clumps of mucus or small particles of tissue. Larger fragments can be placed in fixative for histology | ||
| + | |||
| + | == Nasal aspiration technique == | ||
| + | When a mass is visualised on imaging or rhinoscopy then guided biopsies may be collected. If advanced imaging or rhinoscopy are not available then blind biopsy may still be useful. In one study three techniques (blind biopsy, advanced imaging guided sampling and rhinoscopy guided biopsy) gave similar diagnostic success. In this study approximately one third of diagnoses were made after repeat sampling, therefore, repeat biopsy is recommended if a mass is present but microscopic examination reveals only inflammatory tissue | ||
| + | |||
| + | * Cut the end of a catheter at a 45-degree angle to make a sharp edge (a tom cat catheter may be used). The catheter length is determined by measuring the distance between the external nares and the medial canthus of the eye. A syringe is attached to the catheter, which is then introduced into the nasal cavity until moderate resistance is felt | ||
| + | * Gentle suction is applied while several advances are made with the catheter into the mass. Gentle negative pressure is maintained as the catheter is withdrawn to retain solid tissue fragments | ||
| + | * Alternatively a biopsy forceps attachment during rhinoscopy may be used | ||
| + | * Larger particles can be used to make a touch imprint before being placed in fixative for histology | ||
| + | |||
| + | [[File:NWL Aspirate of nasal carcinoma.jpg|center|thumb|Aspirate of nasal carcinoma ©NationWide Laboratories 2017]] | ||
| + | |||
| + | == Authors & References == | ||
| + | [[NationWide Laboratories]] | ||
Revision as of 17:39, 21 April 2022
Clinical signs associated with nasal disease may include sneezing, nasal or occular discharge, epistaxis, pawing at the nose and facial deformity. Epistaxis may also be seen in
primary or secondary coagulopathies and hyperviscosity syndromes.
Rhinoscopy and cytological evaluation are simple non-invasive procedures which may lead to a definitive diagnosis. Cytological findings must be evaluated with historical, physical, rhinoscopic, radiographic and clinical laboratory findings to maximise the likelihood of achieving a diagnosis.
Negative or non-specific findings do not rule out infection, neoplasia or a foreign body. Surgical exploration of the nasal cavity and biopsy may be required to obtain a definitive diagnosis.
Cytological specimens are collected from the nasal cavity by flushing or aspiration techniques which should be performed after radiography and rhinoscopy so that radiographic detail and visualisation of the cavity is not altered by haemorrhage and manipulation of nasal tissue.
General anaesthesia is required, with an endotracheal tube in place and the cuff inflated to prevent inhalation of blood and fluids during sample collection. Care must be taken not to penetrate the cribriform plate with the instruments used for collecting samples. Some haemorrhage will occur during manipulation of the nasal cavity, but this should not be a complicating factor unless the animal has a coagulopathy, in which case these procedures are contraindicated.
Nasal flushing technique
- Pack the caudal pharynx with absorbent material
- Estimate the depth of the nasal cavity by measuring the distance between the external nares and the medial canthus of the eye. Pass a soft rubber catheter either caudally through the external nares or rostrally from the nasopharynx. Attach a syringe containing approximately 20ml of sterile normal saline, then using alternate positive and negative pressure repeatedly flush the nasal cavity before retrieving fluid and harvest of material. A smaller volume is used in patients <5kg, for example 10ml
- Collect dislodged particulate matter on a gauze sponge caudal to the soft palate or at the nares, depending on the direction of flush. Collect aqueous material by aspiration
- Transfer the fluid into two plain tubes and one EDTA tube. Add 2 drops of formalin to one plain tube and label accordingly. Make a direct air-dried smear of any large clumps of mucus or small particles of tissue. Larger fragments can be placed in fixative for histology
Nasal aspiration technique
When a mass is visualised on imaging or rhinoscopy then guided biopsies may be collected. If advanced imaging or rhinoscopy are not available then blind biopsy may still be useful. In one study three techniques (blind biopsy, advanced imaging guided sampling and rhinoscopy guided biopsy) gave similar diagnostic success. In this study approximately one third of diagnoses were made after repeat sampling, therefore, repeat biopsy is recommended if a mass is present but microscopic examination reveals only inflammatory tissue
- Cut the end of a catheter at a 45-degree angle to make a sharp edge (a tom cat catheter may be used). The catheter length is determined by measuring the distance between the external nares and the medial canthus of the eye. A syringe is attached to the catheter, which is then introduced into the nasal cavity until moderate resistance is felt
- Gentle suction is applied while several advances are made with the catheter into the mass. Gentle negative pressure is maintained as the catheter is withdrawn to retain solid tissue fragments
- Alternatively a biopsy forceps attachment during rhinoscopy may be used
- Larger particles can be used to make a touch imprint before being placed in fixative for histology