Difference between revisions of "Penicillins"
| Line 22: | Line 22: | ||
Over the years different sub-groups of Penicillins have been developed; this is because many bacteria have developed '''beta-lactamases''' which will therefore reduce the action of the antiobiotic | Over the years different sub-groups of Penicillins have been developed; this is because many bacteria have developed '''beta-lactamases''' which will therefore reduce the action of the antiobiotic | ||
| − | + | '''Natural Penicillins''' - benzylpenicillin or Penicillin G | |
| − | + | * Narrow spectrum of activity. | |
| − | + | * Active against gram positive organisms, except beta-lactamase producing Staphylococci. | |
| − | + | * Active against many obligate anaerobes. | |
| − | |||
| − | |||
| + | '''Anti-staphylococcal Penicillins''' - Cloxacillin | ||
| + | * Narrow Spectrum | ||
| + | * Resistant to beta-lactamase producing Staphylococci, and so active against gram positive bacteria. | ||
| + | * Active against many obligate anaerobes. | ||
| + | '''Aminopenicillins''' - Amoxycillin | ||
• Aminopenicillins (e.g. Amoxycillin) were also synthesised to widen the spectrum of action to include many more gram negative organisms than the natural penicillins. The aminopenicillins are still susceptible to hydrolysis by beta-lactamase enzymes. | • Aminopenicillins (e.g. Amoxycillin) were also synthesised to widen the spectrum of action to include many more gram negative organisms than the natural penicillins. The aminopenicillins are still susceptible to hydrolysis by beta-lactamase enzymes. | ||
| Line 39: | Line 42: | ||
• Addition of a beta-lactamase inhibitor (Clavulanic acid) broadens the spectrum of most sub-gps. | • Addition of a beta-lactamase inhibitor (Clavulanic acid) broadens the spectrum of most sub-gps. | ||
| + | |||
| + | e.g. Penicillin sub-families (all have good activity vs. many obligate anaerobic bacteria) | ||
==Pharmacokinetic Considerations== | ==Pharmacokinetic Considerations== | ||
Revision as of 14:05, 22 October 2008
| This article is still under construction. |
|
|
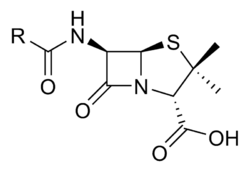
Mechanism of Action
Penicillins are β-Lactam antibiotics. This means that they interfere with the synthesis of peptidoglycan, a crucial component of the bacterial cell wall. Penicillins bind to proteins on the bacteria and then inhibit a transpeptidation enzyme, whose role is to cross-link peptides attached to the peptiglycan backbone. This weakens the bacterial cell wall integrity.
The penicillins also inactivate an inhibitor of autolytic enzymes present in the cell wall, thus resulting in lysis of the bacterium.
Due to the lytic nature of the group of drugs they are considered to be bacteriocidal (ie kill bacteria).
Spectrum of Activity
Over the years different sub-groups of Penicillins have been developed; this is because many bacteria have developed beta-lactamases which will therefore reduce the action of the antiobiotic
Natural Penicillins - benzylpenicillin or Penicillin G
- Narrow spectrum of activity.
- Active against gram positive organisms, except beta-lactamase producing Staphylococci.
- Active against many obligate anaerobes.
Anti-staphylococcal Penicillins - Cloxacillin
- Narrow Spectrum
- Resistant to beta-lactamase producing Staphylococci, and so active against gram positive bacteria.
- Active against many obligate anaerobes.
Aminopenicillins - Amoxycillin
• Aminopenicillins (e.g. Amoxycillin) were also synthesised to widen the spectrum of action to include many more gram negative organisms than the natural penicillins. The aminopenicillins are still susceptible to hydrolysis by beta-lactamase enzymes.
• Extended spectrum penicillins (e.g. Ticarcillin) have been produced to increase efficacy against some of the most difficult gram negative infections to treat, such as Pseudomonas aeruginosa. These drugs are still susceptible to hydrolysis by beta-lactamase.
• Addition of a beta-lactamase inhibitor (Clavulanic acid) broadens the spectrum of most sub-gps.
e.g. Penicillin sub-families (all have good activity vs. many obligate anaerobic bacteria)