Difference between revisions of "Toxoplasmosis - Cat and Dog"
| Line 184: | Line 184: | ||
#Dubey, J P (2005) Toxoplasmosis in cats and dogs. ''Proceedings of the World Small Animal Veterinary Association 2005''. | #Dubey, J P (2005) Toxoplasmosis in cats and dogs. ''Proceedings of the World Small Animal Veterinary Association 2005''. | ||
#Tilley, L.P. and Smith, F.W.K.(2004)'''The 5-minute Veterinary Consult (Fourth Edition)''' ''Blackwell Publishing''. | #Tilley, L.P. and Smith, F.W.K.(2004)'''The 5-minute Veterinary Consult (Fourth Edition)''' ''Blackwell Publishing''. | ||
| − | # Lappin, M R (1996) Feline toxoplasmosis: interpretation of diagnostic test results. ''Seminars in Veterinary Medicine and Surgery'', | + | # Lappin, M R (1996) Feline toxoplasmosis: interpretation of diagnostic test results. ''Seminars in Veterinary Medicine and Surgery'', '''11''', 154-160. |
| − | '''11''', 154-160. | ||
# Dubey, J p and Lappin, M R (1998) Toxoplasmosis and neosporosis. In '''Infectious Diseases of the Dog and Cat''', ''W B Saunders'', 493-503. | # Dubey, J p and Lappin, M R (1998) Toxoplasmosis and neosporosis. In '''Infectious Diseases of the Dog and Cat''', ''W B Saunders'', 493-503. | ||
#Merck & Co (2008) '''The Merck Veterinary Manual (Eighth Edition)''' ''Merial'' | #Merck & Co (2008) '''The Merck Veterinary Manual (Eighth Edition)''' ''Merial'' | ||
Revision as of 10:41, 13 August 2010
Description
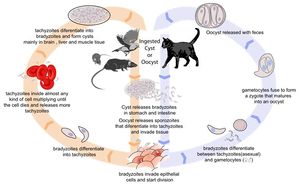
Toxoplasma gondii is an obligate, intracellular coccidian parasite that is capable of infecting most mammals including man. Cats and other Felidae are the definitive host for T. gondii, and all other mammals are intermediate hosts. Toxoplasma gondii has three infectious stages: 1) sporozoites; 2) an actively reproducing stage called tachyzoites; and 3) slowly multiplying bradyzoites. Tachyzoites and bradyzoites are found in tissue cysts, whereas sporozoites are containted within oocysts, which are excreted in the faeces. This means that the protozoa can be transmitted by ingestion of oocyst-contaminated food or water, or by consumption of infected tissue. Transplacental infection is also possible.
Life Cycle
In the naive definitive host, Toxoplasma gondii undergoes an enteroepithelial life cycle. Cats become infected by ingesting intermediate hosts containing tissue cysts, which release their bradyzoites in the gastrointestinal tract when the wall is digested. Bradyzoites then penetrate the small intestinal epithelium and produce five types of schizonts, which then give rise to merozoites. Male and female gamonts are formed from merozoites, which fertilise to form a macrogamont. A wall forms aroung the macrogamont to produce an oocyst, which is passed in the faeces approximately three days after ingestion of the tissue cyst. Initially, these oocysts are unsporulated and are therefore not infectious, but after 1 to 5 days sporulation occurs to produce two sporocysts, each with four infectious sporozoites. This sporulation is dependent on temperature and aeration, and sporocysts can remain viable in the environment for up to 18 months even if exposed to high or freezing temperatures and low humidity. As cats generally develop immunity to T. gondii after the initial infection, they will only shed oocysts once in their lifetime.
When other, non-feline, carnivores (such as dogs) consume tissue cysts or oocysts from cat faeces, Toxoplasma gondii initiates extraintestinal replication. This process is the same for all hosts, and does not vary with the form of the parasite ingested. Bradyzoites and sporozoites, from cysts and oocysts respectively, are released in the intestine and infect the intestinal epithelium where they replicate. This produces tachyzoites, which are lunate in shape, about 6 microns in diameter and possess the ability to multiply in almost any cell type. The infected cell ruptures to release tachyzoites which then disseminate via blood and lymph to infect other tissues. Tachyzoites then replicate intracellularly and, if the cell does not burst, they eventually encyst and persist for the life of the host. Tissue cysts readily form in the CNS, muscles and visceral organs.
Transmission
Although any of the three life stages described above can infect warm-blooded vertebrates, most infections are acquired following the ingestion of sporozoites or bradyzoites, as tachyzoites are easily inactivated in the gastric environment. As cats rarely practice coprophagy, infection is usually acquired through the ingestion of infected intermediate hosts such as rodents. Dogs tend to consume food or water contaminated with oocysts from cat faeces.
If a pregnant queen is naive to Toxoplasma gondii at the time of ingestion, transplacental infection can occur. The outcome of this depends on the stage of gestation. Infection during the first trimester usually has severe consequences, such as stillbirth or abortion; infection during the second or third trimesters are more likely to give rise to an infected foetus. Kittens infected neonatally commonly show interstitial pneumonia, necrotising hepatitis, myocardidits, non-suppurative encephalits and uveitis on post-mortem examination1.
Pathogenesis
The outcome of primary infection depends on the immune status of the host, as well as the location of and degree of injury caused by tissue cysts. Primary infection normally results in chornic disease, where tissue cysts form but clinical signs are not normally apparent. In immunodeficient animals, or in animals with concurrent illness, chronic infections may become symptomatic as the organism is allowed to proliferate. Acute primary infection in these animals can, rarely, prove fatal.
The mechanism of clinical disease in chronic toxoplasmosis is not fully understood, but may be related to low-level tachyzoite replication, or intermittent antigenaemia and parasitemia2 have been detected in experimentally inoculated cats (Burney and others 1999). The pathogenesis of disease could also be associated with immunological reactions against the organism through formation and deposition of immune complexes, and delayed hypersensitivity reactions3.
Signalment
Cats more commonly show clinical disease than dogs. Male cats are predisposed, and the average age of the feline toxoplasmosis patient is 4 years (range: 2 weeks to 16 years)4. There are no breed predilections.
Diagnosis
Clinical Signs
Clinical signs are determined by the site and extent of organ damage by tachyzoites, and may be acute or chronic. Acute signs manifest at the time of initial infection, whereas chronic signs are associated with reactivation of encysted infection during times of immunocompromise.
In cats, disease is most severe in transplacentally infected kittens, which may be stillborn or die before weaning. Those that survive are anorexic and lethargic, with a pyrexia that does not respond to antibiotics. The lungs, liver or CNS may be necrosed, leading to signs such as dyspnoea, respiratory noise, icterus, ascites and neurological signs. Cats infected post-natally most commonly display gastrointestinal and/or respiratory signs. Again, animals may be anorexic and lethargic, with an antibiotic non-responsive fever. Vomiting, diarrhoea, icterus or abdominal effusion may be apparent, and the cat may lose weight. Ocular signs such as uveitis, iritis and detachment of the retina are also common. Neurologic signs are seen in less than 10% of patients 4 and may present as circling, torticollis, anisocoria, seizures, blindness or inco-ordination. Signs progress rapidly in patients suffering acute disease, in whom respiratory and/or CNS involvement is common. Chronic infections tend to follow a slower course.
In young dogs, Toxoplasma gondii infection is usually generalised, causing fever, weight loss and anorexia. Dyspnoea, diarrhoea and vomiting may also be seen. Older animals more commonly experience localised infections which are primarily associated with the neural and muscular systems. When neurological signs are seen, they usually reflect diffuse inflammation of the CNS. For example, dogs might suffer seizures, ataxia, paresis or muscle weakness. Although cardiac involvement occurs, this is not normally clinically significant. Ocular changes are rare, but are similar to those described in cats.
Laboratory Tests
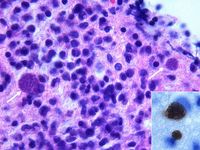
Demonstration of Toxoplasma gondii in the tissues with associated inflammation is required for the definitive diagnosis of clinical toxoplasmosis. For example, tachyzoites may be seen in blood, cerebrospinal fluid, peritoneal and pleural effusions, aqueous humour or transtracheal washes from clinically ill animals. Toxoplasma gondii may also be detected in these samples using PCR, tissue culture or animal inoculation techniques1. These methods may be employed on tissue biopsies too, as well as examination under haematoxylin and eosin or immunohistochemical staining. Immunohistochemistry is preferred to H&E because it is specific for T. gondii. Demonstration of the organism is often most easily achieved post-mortem, as the size of the sample is not restrictive to the likelyhood of seeing T.gondii. In the absence of demonstration of Toxoplasma gondii in the tissues or fluids ante-mortem, there is no one specific test to diagnose toxoplamosis. However, a combination of various diagnostic procedures can be used to build a presumptive diagnosis.
Firstly, clinical signs should be suggestive of toxoplasmosis, despite variation in the presentation of disease between individuals. Although no pathognomic changes for toxoplasmosis are seen on routine haematology, biochemistry and urinalysis, certain results results are often seen in T. gondii infection. For example, most cats show a mild non-regenerative anaemia, and 50% of pateients are initially leukopenic due to lymphopenia. Neutropenia may occur in conjunction with lymphopenia, and leukocytosis may occur during recovery4. Most patients also show and increase in creatine kinase, ALT, SAP, and hypoalbuminaemia is also common1, 4. 25% of cats show hyperbilirubinemia and icterus, and pancreatitis may cause low to low normal serum calcium. A mild proteinuria and bilirubinuria are often revealed by urinalysis.
Demonstration of antibodies in serum, aqueous humour or CSF is indicative of exposure to T. gondii, but does not necessarily show active infection. This could be overcome by testing for T. gondii antigen or immune complexes, but these methods are currently only available to researchers. Several techniques are commercially available for detection of antibody, including ELISA, immunofluorescent antibody testing, western blot immunoassay, Sabin-Feldmann dye test, and agglutination tests. Although these tests are theoretically able to detect all classes of immunoglobulin against Toxoplasma gondii in many species, it seems that feline serum positive for IgM only often reads as a false negative5, 6
A latex agglutination assay and an indirect haemagglutination assay are also available commercially. These assays, which can be used with serum from multiple species, theoretically detect all classes of immunoglobulin directed against T gondii. However, these assays rarely detect antibody in feline serum samples positive only for IgM (Lappin 1996, Dubey and Lappin 1998). A combination of modified agglutination tests using formalin-fixed and acetone-fixed tachyzoites can be used to accurately predict recent infection, but the assays are not commercially available. Using ELISA, approximately 80 per cent of healthy, experimentally infected cats have detectable T gondiispecific IgM in serum within two to four weeks of inoculation with T gondii; the titres are generally negative again by 16 weeks post-infection. Persistent IgM titres (>16 weeks) have been documented commonly in cats coinfected with FIV and in cats with ocular toxoplasmosis. In some chronically infected cats, IgM can be detected again after repeat inoculation with T gondii, primary inoculation with the Petaluma isolate of FIV, and administration of glucocorticoids. Because of these findings, IgM titres cannot accurately predict when a cat is InPractice i NOVEMBER/DECEMBER 1999 583 shedding oocysts or if a cat has clinical toxoplasmosis. However, detectable IgM titres were present in the serum of 93-3 per cent of cats in one study of clinical toxoplasmosis, while IgG titres were only detected in 60 per cent (Lappin and others 1989). Hence, the IgM antibody class appears to correlate more closely to clinical disease than IgG. T gondii-specific IgG can be detected by ELISA in serum in the majority of healthy, experimentally inoculated cats within three to four weeks of infection (Lappin 1996, Dubey and Lappin 1998). IgG antibody titres can be detected for at least six years after infection (Dubey 1995); since the organism probably persists for life, IgG antibodies probably do as well. Single, high IgG titres do not necessarily suggest recent or active infection - healthy cats commonly have titres of above 10,000 six years after experimental induction of toxoplasmosis. The demonstration of an increasing IgG titre can document recent or active disease but, in experimentally infected cats, the time span from the first detectable positive IgG titre to the maximal IgG titre is approximately two to three weeks. Many cats with sublethal clinical toxoplasmosis have chronic, mild clinical signs and may not be evaluated serologically until their IgG antibody titres have reached their maximal values. In humans and cats with reactivation of chronic toxoplasmosis, IgG titres rarely increase. Western blot immunoassay can be used to determine the T gondii antigens recognised by humoral immune responses. Transplacentally infected kittens could be distinguished from T gondii-naive kittens with antibodies in serum from colostrum ingestion by comparing antigen recognition patterns between the queen and kittens.
- Demonstration of an IgM titre of above 1:64 or a
fourfold or greater increase in IgG titre, or the documentation of local antibody production or DNA in aqueous humour or CSF (suggests recent or active infection); Detection of T gondii-specific antibodies produced by the eyes or CNS can be used to document clinical toxoplasmosis. While IgG and IgA class antibodies are produced transiently by the eyes and CNS of healthy cats after experimental inoculation, IgM has only been detected in the aqueous humour or CSF of cats with clinical disease (Lappin 1996, Dubey and Lappin 1998). Most cats with local production of T gondii-specific antibodies in aqueous humour have responded to the administration of anti- Toxoplasma drugs, suggesting that aqueous humour antibody testing can aid in the diagnosis of clinical ocular toxoplasmosis in cats (Lappin and others 1992b). T gondii DNA has been detected in the aqueous humour of cats with uveitis by polymerase chain reaction (PCR) (Lappin and others 1996). However, the organism is also detected occasionally in the aqueous humour of cats without uveitis and so positive results do not provide a definitive diagnosis of clinical toxoplasmosis (Burney and others 1998).
- Faecal examination
T gondii oocysts are 10 x 12 ,um in size and can be demonstrated microscopically in feline faeces following 0 2 4 6 8 12 16 20 26 34 Weeks after inoculation Temporal appearance of T gondii-specific IgM, IgG and IgA antibodies in the serum of experimentally flotation using solutions with a specific gravity of 1-18. inoculated cats Oocysts of the non-pathogenic coccidians Hammondia hammondi and Besnoitia darlingi cannot be distinguished microscopically from those of T gondii; definitive diagnosis relies on laboratory animal inoculation. Most cats with clinical toxoplasmosis have completed the oocyst shedding period and so the diagnostic utility of faecal examination is limited. However, due to the potential zoonotic risk, a faecal examination should be performed for any cat with clinical signs referable to toxoplasmosis.
- Positive response to appropriate treatment (see
below).
Diagnostic Imaging
Radiographs of the thorax in pulmonic toxoplasmosis commonly show patchy alveolar and interstitial pulmonary patterns, but pleural effusions are rare1. Abdominal radiographs can show a variety of changes, including hepatomegaly, pertitoneal effusions, lymphadenopathy, intestinal masses, or pancreatitis (seen as reduced contrast in the right cranial quadrant)1,3. Myelography, CT or MRI can detect mass lesions in cats with CNS involvement.
Pathology
Treatment
The toxoplasmosis patient does not usually require hospitalisation, unless they are suffering severe disease or cannot maintain adqequate nutrition or hydration unaided. Patients showing neurological signs should also be confined and monitored.
Prevention
- Cat
- Impossible if cat is allowed outdoors due to hunting
- If kept indoors, only canned food should be fed and vermin controlled
- ELISA to check if seropositive
For animals other than humans, treatment is seldom warranted. Sulfadiazine (15-25 mg/kg) and pyrimethamine (0.44 mg/kg) act synergistically and are widely used for treatment of toxoplasmosis. While these drugs are beneficial if given in the acute stage of the disease when there is active multiplication of the parasite, they will not usually eradicate infection. These drugs are believed to have little effect on the bradyzoite stage. Certain other drugs, including diaminodiphenylsulfone, atovaquone, and spiramycin are also used to treat toxoplasmosis in difficult cases. Clindamycin is the treatment of choice for dogs and cats, at 10-40 mg/kg and 25-50 mg/kg respectively, for 14-21 days.
Zoonosis
T gondii is an important zoonotic agent. In some areas of the world, up to 60% of the human population have serum IgG titers to T gondii and are likely to be persistently infected. Toxoplasmosis is a major concern for people with immune system dysfunction (eg, people infected with human immunodeficiency virus). In these individuals, toxoplasmosis usually presents as meningoencephalitis and results from the emergence of T gondii from tissue cysts located in the brain as immunity wanes rather than from primary T gondii infection. Toxoplasmosis is also a major concern for pregnant women because tachyzoites can migrate transplacentally and cause birth defects in human fetuses. Infection of women with T gondii may occur after ingestion of undercooked meat or accidental ingestion of oocysts from cat feces. To prevent infection, the hands of people handling meat should be washed thoroughly with soap and water after contact, as should all cutting boards, sink tops, knives, and other materials. The stages of T gondii in meat are killed by contact with soap and water. T gondii organisms in meat can also be killed by exposure to extreme cold or heat. Tissue cysts in meat are killed by heating the meat throughout to 67°C or by cooling to -13°C. Toxoplasma in tissue cysts are also killed by exposure to 0.5 kilorads of gamma irradiation. Meat of any animal should be cooked to 67°C before consumption, and tasting meat while cooking or while seasoning should be avoided. Pregnant women should avoid contact with cat litter, soil, and raw meat. Pet cats should be fed only dry, canned, or cooked food. The cat litter box should be emptied daily, preferably not by a pregnant woman. Gloves should be worn while gardening. Vegetables should be washed thoroughly before eating because they may have been contaminated with cat feces.
At present there is no vaccine to prevent toxoplasmosis in humans.
Prognosis
Links
- Cornell College of Veterinary Medicine Toxoplasmosis Factsheet
- Feline Advisory Bureau: Toxoplasmosis in cats and man
- The Merck Veterinary Manual - Toxoplasmosis
References
- Lappin, M (1999) Feline toxoplasmosis. In Practice, 21(10), 578-589.
- Burney, D P et al (1999) Detection of Toxoplasma gondii parasitemia in experimentally inoculated cats. Journal of Parasitology, 85.
- Dubey, J P (2005) Toxoplasmosis in cats and dogs. Proceedings of the World Small Animal Veterinary Association 2005.
- Tilley, L.P. and Smith, F.W.K.(2004)The 5-minute Veterinary Consult (Fourth Edition) Blackwell Publishing.
- Lappin, M R (1996) Feline toxoplasmosis: interpretation of diagnostic test results. Seminars in Veterinary Medicine and Surgery, 11, 154-160.
- Dubey, J p and Lappin, M R (1998) Toxoplasmosis and neosporosis. In Infectious Diseases of the Dog and Cat, W B Saunders, 493-503.
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition) Merial
- Fisher, M (2002) Endoparasites in the dog and cat: 2. Protozoa. In Practice, 24(3), 146-153.
- Quinn, P J and McCraw, B M (1972) Current status of toxoplamsa and toxoplasmosis: A review. The Canadian Veterinary Journal, 13(11), 247-262.