Difference between revisions of "Erythrocytes"
Fiorecastro (talk | contribs) |
|||
| (14 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Image:LH Erythrocyte Histology.jpg|thumb|right|150px|<p>'''Erythrocytes'''</p | + | [[Image:LH Erythrocyte Histology.jpg|thumb|right|150px|<p>'''Erythrocytes'''</p>]] |
Also known as '''''red blood cells (RBCs) | Also known as '''''red blood cells (RBCs) | ||
<p>Erythrocytes deliver oxygen to, and remove carbon dioxide from tissues.</p> | <p>Erythrocytes deliver oxygen to, and remove carbon dioxide from tissues.</p> | ||
==Development== | ==Development== | ||
Erythrocytes are derived from the stem cell ([[Haematopoiesis - Overview#Colony Forming Units|CFU-GEMM]]) and formed in a process known as [[Erythropoiesis|erythropoiesis]]. | Erythrocytes are derived from the stem cell ([[Haematopoiesis - Overview#Colony Forming Units|CFU-GEMM]]) and formed in a process known as [[Erythropoiesis|erythropoiesis]]. | ||
| − | ==Structure== | + | ==Erythrocyte Structure== |
| − | [[Image:LH_Avian_Erythrocyte_Histology.jpg|thumb|right|150px|<p>'''Avian Erythrocytes'''</p | + | [[Image:LH_Avian_Erythrocyte_Histology.jpg|thumb|right|150px|<p>'''Avian Erythrocytes'''</p>]] |
===Mammals=== | ===Mammals=== | ||
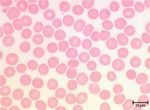
<p>Erythrocytes are small (4-8µm), circular, anucleate biconcave cells that lack organelles. </p> | <p>Erythrocytes are small (4-8µm), circular, anucleate biconcave cells that lack organelles. </p> | ||
| Line 16: | Line 16: | ||
==Function== | ==Function== | ||
| − | RBCs contain haemoglobin, an oxygen binding protein that is responsible for the transport of oxygen from the lungs to tissues, and the transportation of carbon dioxide from the tissues to the lungs for elimination. | + | RBCs contain '''haemoglobin''', an oxygen binding protein that is responsible for the transport of oxygen from the lungs to tissues, and the transportation of carbon dioxide from the tissues to the lungs for elimination. |
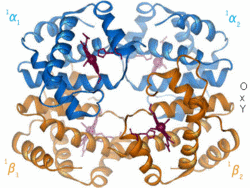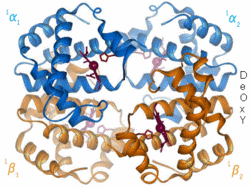
[[Image:LH Hb Animation.gif|thumb|right|250px|<p>Blue(α) & orange(β) are globin chains</p><p>Red objects represent haem groups</p>]] | [[Image:LH Hb Animation.gif|thumb|right|250px|<p>Blue(α) & orange(β) are globin chains</p><p>Red objects represent haem groups</p>]] | ||
| − | <p>Haemoglobin (Hb)is a metalloprotein that binds oxygen reversibly and accounts for almost all of the protein in the erythrocyte.</p> | + | <p>Haemoglobin (Hb) is a metalloprotein that binds oxygen reversibly and accounts for almost all of the protein in the erythrocyte.</p> |
<p>When bound to oxygen it is referred to as oxyhaemoglobin and when unbound, deoxyhaemoglobin. Haem gives blood its colour, and this colour depends on its saturation. When fully saturated with four oxygen molecules it appears red and when completely unsaturated it appears blue-red.</p> | <p>When bound to oxygen it is referred to as oxyhaemoglobin and when unbound, deoxyhaemoglobin. Haem gives blood its colour, and this colour depends on its saturation. When fully saturated with four oxygen molecules it appears red and when completely unsaturated it appears blue-red.</p> | ||
<p>Haemoglobin also acts as an extracellular buffer | <p>Haemoglobin also acts as an extracellular buffer | ||
| Line 26: | Line 26: | ||
**pK<sub>a</sub> = 7.8 | **pK<sub>a</sub> = 7.8 | ||
</p> | </p> | ||
| − | ====Structure==== | + | ====Haemoglobin Structure==== |
Haemoglobin is composed of globin and four haem groups which contain an iron atom in each haem group. Four oxygen molecules can bind to each haemoglobin - one to each heam group via the iron when in its ferrous state. | Haemoglobin is composed of globin and four haem groups which contain an iron atom in each haem group. Four oxygen molecules can bind to each haemoglobin - one to each heam group via the iron when in its ferrous state. | ||
=====Globin===== | =====Globin===== | ||
| Line 34: | Line 34: | ||
====Types==== | ====Types==== | ||
| − | <p> There are three types of haemoglobin: embryonic, | + | <p> There are three types of haemoglobin: embryonic, foetal and adult. Embryonic is present in the early stages of foetal development. Foetal haemoglobin has a higher affinity for oxygen than adult haemoglobin and is replaced by adult haemoglobin shortly after birth. Dogs do not have foetal or embryonic haemoglobin. Horses do not have embryonic haemoglobin and their foetal haemoglobin is structurally identical to adult haemoglobin and as such some would say that there is no foetal haemoglobin. Pig (and horse) foetal haemoglobin has the same affinity for oxygen as their adult haemoglobin.</p> |
| − | ==== | + | ====Chemistry==== |
<p> | <p> | ||
*One gram of haemoglobin binds 1.34g of oxygen. | *One gram of haemoglobin binds 1.34g of oxygen. | ||
| Line 50: | Line 50: | ||
Erythrocytes have a limited life span and as they age their membranes become more fragile leading to a loss of function and swelling. Damage occurs as the fragile cells pass through narrow capillaries which occurs particularly in the [[Spleen - Anatomy & Physiology#Erythrocytes & Platelets|spleen]]. Ageing also causes sialic acid to be lost from the membrane and this exposes galactose moieties leading to phagocytosis. | Erythrocytes have a limited life span and as they age their membranes become more fragile leading to a loss of function and swelling. Damage occurs as the fragile cells pass through narrow capillaries which occurs particularly in the [[Spleen - Anatomy & Physiology#Erythrocytes & Platelets|spleen]]. Ageing also causes sialic acid to be lost from the membrane and this exposes galactose moieties leading to phagocytosis. | ||
===Life Span=== | ===Life Span=== | ||
| − | The life span of the erythrocyte varies from 70-160 days in | + | The life span of the erythrocyte varies from 70-160 days in domestic species although the life span is often shorter in juvenile animals (e.g. calves and lambs) compared to adult animals. Erythrocytes in small animals have a shorter life span than in the larger domestic species. |
{| border="0" cellpadding="5" cellspacing="1" | {| border="0" cellpadding="5" cellspacing="1" | ||
!style="color:black;background-color:#FFC1C1;" colspan="7"|Life span (days) | !style="color:black;background-color:#FFC1C1;" colspan="7"|Life span (days) | ||
| Line 75: | Line 75: | ||
Around 90% of erythrocytes are phagocytised by [[Macrophages|macrophages]] in the [[Liver - Anatomy & Physiology|liver]], [[Spleen - Anatomy & Physiology|spleen]] and [[Bone Marrow - Anatomy & Physiology|bone marrow]], with the other 10% breaking down in circulation. | Around 90% of erythrocytes are phagocytised by [[Macrophages|macrophages]] in the [[Liver - Anatomy & Physiology|liver]], [[Spleen - Anatomy & Physiology|spleen]] and [[Bone Marrow - Anatomy & Physiology|bone marrow]], with the other 10% breaking down in circulation. | ||
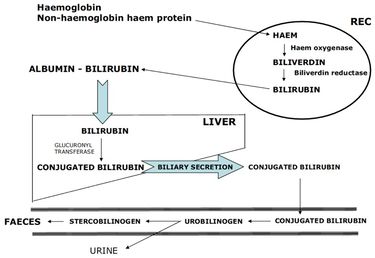
| − | Following phagocytosis, haemoglobin is broken down into haem and globin. Globin, a protein, is hydrolysed into amino acids while the haem is transformed into biliverdin and then into bilirubin before being excreted into the blood stream bound to albumin. In the [[Liver - Anatomy & Physiology|liver]] it is conjugated and excreted into bile which in turn is excreted into the [[Alimentary System Overview - Anatomy & Physiology#Stomach| | + | Following phagocytosis, haemoglobin is broken down into haem and globin. Globin, a protein, is hydrolysed into amino acids while the haem is transformed into biliverdin and then into bilirubin before being excreted into the blood stream bound to albumin. In the [[Liver - Anatomy & Physiology|liver]] it is conjugated and excreted into bile which in turn is excreted into the [[Alimentary System Overview - Anatomy & Physiology#Stomach|gastro-intestinal system]]. The iron from haem is either stored as insoluble ferritin in the [[Liver - Anatomy & Physiology|liver]] (in hepatocytes and [[Macrophages|macrophages]]) or it is directly transported to the [[Bone Marrow - Anatomy & Physiology|bone marrow]] for reuse in [[Erythropoiesis|erythropoiesis]]. |
| − | [[Image:Haem Breakdown.jpg|center|thumb| | + | [[Image:Haem Breakdown.jpg|center|thumb|375px|<div style="text-align: center;"><p>'''Haem breakdown pathway'''</p><sup>©Nottingham Uni 2008</sup></div>]] |
<br> | <br> | ||
| + | |||
| + | {{Template:Learning | ||
| + | |powerpoints = [[Blood and Haemopoiesis resource|Tutorial about histology of blood cells]] | ||
| + | }} | ||
| + | |||
| + | |||
| + | |||
| + | ==Webinars== | ||
| + | <rss max="10" highlight="none">https://www.thewebinarvet.com/clinical-pathology/webinars/feed</rss> | ||
| + | |||
| + | [[Category:Blood Cells]] [[Category:Kate English reviewing]] | ||
Latest revision as of 16:30, 5 January 2023
Also known as red blood cells (RBCs)
Erythrocytes deliver oxygen to, and remove carbon dioxide from tissues.
Development
Erythrocytes are derived from the stem cell (CFU-GEMM) and formed in a process known as erythropoiesis.
Erythrocyte Structure
Mammals
Erythrocytes are small (4-8µm), circular, anucleate biconcave cells that lack organelles.
The cell's membrane is a lipid bilayer which contains two different protein groups. Integral membrane proteins, which make up most of the proteins, and peripheral membrane proteins. The arrangement of these proteins makes the membrane elastic but stable and contributes towards the biconcave shape.
The biconcave shape increases the cell's surface area by 20-30%, thereby increasing the probability of oxygen binding with the haemoglobin molecule within the RBC.
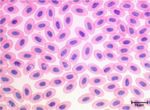
Birds
In birds erythrocytes are ovoid and contain a nucleus.
Function
RBCs contain haemoglobin, an oxygen binding protein that is responsible for the transport of oxygen from the lungs to tissues, and the transportation of carbon dioxide from the tissues to the lungs for elimination.
Haemoglobin (Hb) is a metalloprotein that binds oxygen reversibly and accounts for almost all of the protein in the erythrocyte.
When bound to oxygen it is referred to as oxyhaemoglobin and when unbound, deoxyhaemoglobin. Haem gives blood its colour, and this colour depends on its saturation. When fully saturated with four oxygen molecules it appears red and when completely unsaturated it appears blue-red.
Haemoglobin also acts as an extracellular buffer
- Hb(O2)H + H2 ↔ Hb(O2)- + H3O+
- pKa = 6.6
- HbH + H2O ↔ Hb- + H3O+
- pKa = 7.8
Haemoglobin Structure
Haemoglobin is composed of globin and four haem groups which contain an iron atom in each haem group. Four oxygen molecules can bind to each haemoglobin - one to each heam group via the iron when in its ferrous state.
Globin
Globin is composed of two polypeptide groups each composed of two polpeptides chains. The chains are identical within the group, but differ between the groups. Globin varies between species and individuals within a species due to changes in the polypeptide chain sequence.
Haem
Each of the haem groups has an iron atom at its centre and each iron atom binds one molecule of oxygen. Haem groups are identical in all species.
Types
There are three types of haemoglobin: embryonic, foetal and adult. Embryonic is present in the early stages of foetal development. Foetal haemoglobin has a higher affinity for oxygen than adult haemoglobin and is replaced by adult haemoglobin shortly after birth. Dogs do not have foetal or embryonic haemoglobin. Horses do not have embryonic haemoglobin and their foetal haemoglobin is structurally identical to adult haemoglobin and as such some would say that there is no foetal haemoglobin. Pig (and horse) foetal haemoglobin has the same affinity for oxygen as their adult haemoglobin.
Chemistry
- One gram of haemoglobin binds 1.34g of oxygen.
- Haemoglobin concentration (g/l) varies between species
- Poultry: 90
- Ruminants: 100-120
- Dogs: 150
Antigens
The RBC surface contains glycoproteins that are specific to the individual and potentially antigenic. Plasma contains antibody like substances known as agglutins - mixing RBCs with the blood of another individual can create an agglutination reaction where the plasma agglutins and the RBC glycoproteins bind together. This is the basis of a transfusion reaction and other blood type abnormalities associated with reproduction such as neonatal isoerythrolysis in horses and fading kitten syndrome.
Degradation
Erythrocytes have a limited life span and as they age their membranes become more fragile leading to a loss of function and swelling. Damage occurs as the fragile cells pass through narrow capillaries which occurs particularly in the spleen. Ageing also causes sialic acid to be lost from the membrane and this exposes galactose moieties leading to phagocytosis.
Life Span
The life span of the erythrocyte varies from 70-160 days in domestic species although the life span is often shorter in juvenile animals (e.g. calves and lambs) compared to adult animals. Erythrocytes in small animals have a shorter life span than in the larger domestic species.
| Life span (days) | ||||||
|---|---|---|---|---|---|---|
Cat
|
Pig
|
Dog
|
Goat
|
Horse
|
Sheep
|
Cattle
|
70
|
85
|
120
|
125
|
145
|
150
|
160
|
Transfused dog erythrocytes last around 21 days
Breakdown
Around 90% of erythrocytes are phagocytised by macrophages in the liver, spleen and bone marrow, with the other 10% breaking down in circulation.
Following phagocytosis, haemoglobin is broken down into haem and globin. Globin, a protein, is hydrolysed into amino acids while the haem is transformed into biliverdin and then into bilirubin before being excreted into the blood stream bound to albumin. In the liver it is conjugated and excreted into bile which in turn is excreted into the gastro-intestinal system. The iron from haem is either stored as insoluble ferritin in the liver (in hepatocytes and macrophages) or it is directly transported to the bone marrow for reuse in erythropoiesis.
| Erythrocytes Learning Resources | |
|---|---|
 Selection of relevant PowerPoint tutorials |
Tutorial about histology of blood cells |
Webinars
Failed to load RSS feed from https://www.thewebinarvet.com/clinical-pathology/webinars/feed: Error parsing XML for RSS