Major Histocompatability Complexes
Revision as of 13:25, 9 September 2008 by Nabrown (talk | contribs) (→The Genetics of the MHC (Polymorphism))
|
|
Introduction
T-cells rely on Major Histocompatability Complexes (MHC) to present antigen fragments for their recognition. MHC has evolved to form two classes for antigen presentation: MHC I presents digestion fragments from antigen in cellular cytoplasm, and MHC II presents digestion fragments from antigen in the tissue fluid. As such, MHC I tends to bind slightly smaller peptides (~9 amino acids) than MHC II (~15 amino acids).
Classes
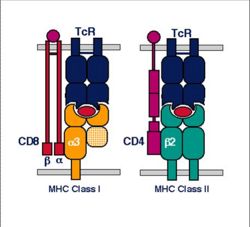
MHC I
Structure
- MHC class I is expressed on virtually all nucleated cells
- MHC class I consists of a membrane-associated heavy chain bound non-covalently with a secreted light chain
- Heavy chain:
- Made up of three distinct extracellular protein domains
- α1, α2 and α3
- The C- terminus is cytoplasmic
- Made up of three distinct extracellular protein domains
- Light chain:
- Known as β2-microglobulin
- Similar in structure to one of the heavy chain domains
- Not membrane associated
- But binds to the α3-domain of the heavy chain
- Heavy chain:
- The MHC class I domains are structurally and genetically related to immunoglobulin and TcR domains
- The outer domains (α1 and α2) are like the variable domains
- The α3 domain and β2m are like thrconstant domains
- MHC class I molecules are folded to form specific 3-dimensional structures
- The α1 and α2 domains are folded to produce an antigen-binding groove
- This groove can bind molecules of a limited size only (8-10 amino acids)
- This limits the size of epitope seen by the T-cell receptors
- This groove can bind molecules of a limited size only (8-10 amino acids)
- The α1 and α2 domains are folded to produce an antigen-binding groove
Presentation Pathway
- MHC I presents endogenous (that is, intracellular) peptides
- Viral proteins are broken down to peptides by the proteasome and transferred to the endoplasmic reticulum via TAP (Transporters associated with Antigen Processing) molecules
- In the ER peptides are processed with empty MHC I molecules and exported to the cell surface for presentation
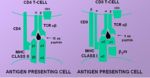
- MHC class I molecules present these to the T-cell receptors of CD8+ T-cells
MHC II
Structure
- MHC class II is expressed mainly on macrophages, dendritic cells and B-lymphocytes
- MHC class II consists of membrane-associated α and β chains
- Each chain is a transmembrane glycoprotein
- The extracellular parts of each chain have two Ig-like domains
- α1 and 7alpha;2, β1 and β2
- The outer domains (α1 and β1) are variable-like
- The inner domains (α2 and β2) are constant-like
- α1 and 7alpha;2, β1 and β2
- The 3-dimensional structure of MHC class II is similar to MHC class I
- The outer domains of the α and β chains fold in a similar way to the α1 and α2 domains of class I
- Produce the antigen-binding groove
- The outer domains of the α and β chains fold in a similar way to the α1 and α2 domains of class I
Presentation Pathway
- MHC II presents exogenous (that is, derived from the ECF) peptides
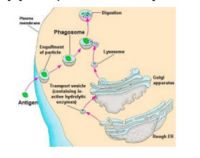
- Endocytosed antigen interacts with MHC II in the cytoplasm to form a complex:
- Antigen is endoycotsed from the ECF
- Lysosomes fuse with primary endosomes to digest the antigen to peptides
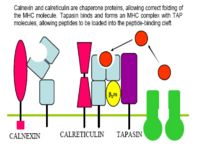
- MHC II is meanwhile being produced by the endoplasmic reticulum, along with an invariant chain chaperone
- These pathways (endoytotic and secretory) merge to allow interaction between the antigen and MHC II:
- The invariant chain is digested, leaving a CLIP peptide in the binding groove
- Foreign antigen then replaces the CLIP peptide
- The MHC II-antigen complex is then secreted to the cell surface for presentation to CD4+ T-cells
Interaction of MHC With Antigen
- The MHC molecules do not recognise specific amino acid sequences of antigens
- Instead, they recognise particular motifs of amino acids
- The association of any MHC allele with a peptide may be determined by the presence of as few as two amino acids
- However, these determinants must be present within a particular array
- The actual identity of the amino acids in not important for MHC binding
- Instead, the physical and chemical characteristics of the amino acid are vital
- Interactions of individual amino acids at the head and tail of the peptide-binding groove control the binding of peptides
- Are mainly positioned at the floor of the antigen-binding groove, or within the helices facing into the groove
- These MHC amino acids associate with amino acids near the ends of the peptides
- The intervening stretch of peptide folds into a helix within the groove
- Is the target for T cell receptor recognition
- MHC molecules have the capacity to bind to trillions of different peptides
- Adopt a flexible floppy conformation until a peptide binds
- Folds around the peptide to increase stability of the complex
- Uses a small number of anchor residues to tether the peptide allowing different sequences between anchors and different lengths of peptides to bind
TCR-MHC Interaction
- Only peptide associated with self-MHC will interact with and activate T cells
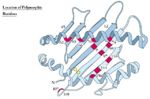
The Genetics of the MHC (Polymorphism)
- Each individual has 6 types of MHC
- MHC molecules are co-dominantly expressed
- The combination of alleles in a chromosome is called an MHC Haplotype
- Different individuals have different critical amino acids within the MHC
- I.e. different amino acids that determine peptide binding
- This variation is termed MHC polymorphism
- Each polymorphic variant is called an allele
- Both type I and type II MHC molecules are highly polymorphic
- Most polymorphic regions of class I are in the alpha 1 and alpha 2 domains
- Most polymorphic regions of class II are in the alpha 1 and beta 1 domains
- Most polymorphisms are point mutations
- There are millions of variations in antibodies and TCR
- However, with MHC there is very limited variation between molecules
- Allelic variation within the MHC molecule occurs at the peptide binding site and on the top or sides of the binding cleft
- Polymorphisms and polygenism in the MHC protects the population from pathogens evading the immune system
- MHC polymorphism has been best studied in the human
In the Human
- Humans express:
- Three types (loci) of MHC class I molecules
- HLA (Human Leukocyte Antigen)- A, B, and C
- Three loci of MHC class II molecules
- HLA-DP, DQ and DR
- Three types (loci) of MHC class I molecules
- In the entire human population there are only approximately 50 different variants (alleles) at each MHC class I and class II locus
- The variation within MHC class I is entirely on the class I heavy chain
- The β2m is invariant
- The variation within MHC class II is mainly within the β chains
- The variation within MHC class I is entirely on the class I heavy chain
- Every individual has two alleles at each MHC locus
- One inherited from each parent
- Any individual will therfore express two variants at most at each locus
- This gives a maximum variability for an individual of:
- 6 different variants of MHC class I
- 2 each of HLA- A, B and C
- 6 different variants of MHC class II
- 2 each of HLA- DP, DQ and DR
- 6 different variants of MHC class I
- This gives a maximum variability for an individual of:
- Many animal species have fewer loci than the human
- E.g. ruminants have no MHC class II DP
MHC and Disease
- Antigen from a pathogen has to be seen by the host MHC before an efficient immune response can occur
- There is therefore a constant evolutionary battle between the host and the pathogen
- There is selective pressure on the pathogen to evolve proteins that do not interact with the host MHC
- There is selective pressure on the host to continue to recognize the pathogen
- There is therefore a constant evolutionary battle between the host and the pathogen
- The consequence of this parallel evolution is that host-pathogen relationships can lead to the selection of particular MHC variants, for example:
- MHC class II alleles DR13/DR1*1301 are prevalent in Central and Western Africa
- Impart resistance to malaria
- MHC-DRB1 is prevalent in Western Europe, but rare in the Inuit populations of North America
- Associated with the clearance of hepatitis B infection in Western Europe
- Inuits have the highest incidence of hepatitis B in the world
- In humans there are also strong associations between certain alleles and some autoimmune diseases, for example:
- Diabetes mellitus
- Ankylosing spondylitis
- Rheumatoid arthritis
- MHC class II alleles DR13/DR1*1301 are prevalent in Central and Western Africa