Difference between revisions of "Mycobacterium bovis"
| Line 104: | Line 104: | ||
[[Category:Cattle Bacteria]][[Category:Respiratory Diseases - Cattle]] | [[Category:Cattle Bacteria]][[Category:Respiratory Diseases - Cattle]] | ||
[[Category:Mycobacterium species]] | [[Category:Mycobacterium species]] | ||
| − | [[category:Expert_Review]] | + | [[category:Expert_Review - Farm Animal]] |
[[Category:Respiratory_Bacterial_Infections]] | [[Category:Respiratory_Bacterial_Infections]] | ||
[[Category:Zoonoses]] | [[Category:Zoonoses]] | ||
Revision as of 22:49, 5 March 2011
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Also known as: Bovine TB — Bovine Tuberculosis
Introduction
Mycobacterium bovis causes tuberculosis in cattle. It is a chronic disease characterised by granulomatous nodular lesions in any organ, although the respiratory system is most commonly affected. The nodules often become necrotic with a caseous centre. The primary lesions may disseminate to involve other body systems.
Inhalation of ruminal gases is the most common route of entry for the mycobacterium organism, and spread of the disease is usually via cow-to-cow contact via aerosol. Cattle can also become infected by ingestion of the causative agent; this is the usual route of entry when the badger is involved, by infecting grazing land or water troughs. Calves with infected dams can become affected via the milk, and intrauterine infection at coitus has been reported.
A higher level of infection is required to establish the alimentary form of the disease and this is reflected by its lower incidence in comparison to the respiratory form. In the respiratory form, the mycobacteria are inhaled, gaining entry to the respiratory system. The organisms are phagocytosed by alveolar macrophages where they multiple and result in a characteristic granulomatous inflammation and tubercle formation. Ultimately, caseous necrosis develops and the nodule ruptures, releasing the organism and infection spreads. The cell-mediated immune system is activated and produces cytotoxic T-lymphocytes, which attack and destroy infected cells, leading to a type IV hypersensitivity reaction.
Historically bovine TB was a major cause of human TB, but the introduction of tuberculin testing and slaughter, meat inspection at abattoirs, the pasteurisation of milk and the BCG vaccination has dramatically reduced transmission to humans and bovine TB as a cause of human disease is now very low indeed.
The disease is of serious economic importance to farmers because of the stringent control measures, which remain in place. These include the slaughter of infected animals and movement restrictions placed on farms with reactors or inconclusive results.
Pathogenesis and Pathogenicity
The ability of mycobacteria to survive and multiply within macrophages determines whether disease will occur within the host. Survival and multiplication of the organisms in macrophages at primary site of infection happens due to prevention of phagosome-lysosome fusion. Mycobacteria utilize several virulence factors including cord factor or trehalose dimycolate, surface glycolipid, sulfatides, lipoarabinomannan, heteropolysaccharide, heat shock protein, complement, and tubuloprotein. The types of immune responses that are critical in responding to mycobacterial infection are cell-mediated immunity and the delayed hypersensitivity response.
Pathogenicity of mycobacteria depends on their ability to escape phagocytic killing, mostly imparted by the cell wall consitiutents:
- Cord factor (trehalose dimycolate) – surface glycolipid responsible for serpentine growth in vitro.
- Suphatides – surface glycolipid containing sulphur which prevents fusion of phagosome with lysosome. cAMP secreted by the bacteria may also facilitate this.
- LAM – heteropolysaccharide which inhibits macrophage activation by IFNγ and induces macrophages to secrete TNFα which induces fever and IL-10 which suppresses mycobacteria-induced T cell proliferation.
- The wax of the cell wall, peptidoglycans and other glycolipids are responsible for the adjuvant activity – attracts antigen presenting cells.
- Tubuloprotein – important antigen; purified tubuloprotein is the basis of the tuberculin test.
Mycobacteria are released from macrophages and also migrate within macrophages around the body. Waxy cell wall contributes to the host immune response to the mycobacteria and the development of lesions. The host mounts a cell-mediated immune response with activated macrophages and sensitised T cells followed by a delayed-type hypersensitivity response with granuloma formation.
Signalment
The disease usually affects heifers or young stock but cases can occur in cattle of any age. TB is more common in dairy herds.
Incidence of the disease has increased over the past 15 years; it is prevalent in Wales and the southwest of England but is re-emerging in other parts of the UK such as the west Midlands and northwest England.
Most warm-blooded animals are susceptible to bovine TB and can act as a reservoir for infection. The disease in cattle has been associated with wildlife species in a number of countries; the European badger and red deer in the UK, opossums and ferrets in New Zealand, mule deer, white-tailed deer, elk, and bison in North America and water buffalo in Australia.
Diagnosis
The intradermal comparative tuberculin test is widely used in the UK for diagnosis of the disease. Two injections are given subcutaneously in the neck of cattle, an avian and a bovine tuberculin purified protein derivative (PPD). The thickness of the skin is recorded at each injection site. The test is read after 72 hours, and the thickness of the skin is remeasured. Interpretation is based on finding a swelling or increase in skin thickness at the site of the injection. A comparison must be made between the reaction to avian and the bovine tuberculin to account for cross reactivity with related diseases, such as atypical mycobacteriosis, or Johne's disease.
A single intradermal test is used in many countries but has the disadvantage of giving reactors to avian tuberculosis and Johne's disease.
Clinical Signs
Location and severity depend on which body systems are affected and the advancement of the disease. Due to the testing and slaughter policy most cases in the UK are identified before development of clinical signs.
Respiratory form
- Chronic cough- soft and productive
- Tachypnoea
- Dyspnoea
- Dull areas on auscultation of the lungs in advanced cases
Alimentary form
- Few clinical signs
- Diarrhoea
- Bloat
Mammary involvement is now rarely seen; the uterine form is also uncommon but may result in abortion.
Laboratory Tests
An ELISA test has been developed but is not widely used. The gamma interferon test can also be used for diagnosis of the condition.
Laboratory examination of lesions, lymph nodes and milk can be performed. Lesions contain macrophages, multinucleate giant cells and later a central area of caseous necrosis, giving a cheesy appearance. Tissues can be prepared using Ziehl-Neelson stain. Bacterial isolation requires Lowenstein-Jensen medium.
Pathology
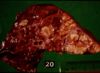
Findings at post mortem depend on the route of entry of the organism, whether it became generalised or not and the stage of the disease. One or more lymph nodes will display the characteristic granulomatous tubercles. In the respiratory form the mediastinal and bronchial lymph nodes are affected, with lesions in the lungs.
If the mycobacteria disseminated from the primary complex then lymph nodes in other regions will also be affected and there will be multiple small foci of infection on other organs.
Microscopically there are epithelioid cells, with large vesicular nuclei and pale cytoplasm and giant cells, formed by the fusion of macrophages, are found at the centre of tubercles. Surrounding this there is a narrow layer of lymphocytes, mononuclear cells and plasma cells, more advanced cases show peripheral fibroplasia and central necrosis.
Treatment and Control
Treatment is not usually an option due to the chronic nature of the disease, zoonotic potential and test and slaughter policy.
Control in many countries is centred on tuberculin testing with slaughter of reactors and movement restrictions to the premises. Research work continues into the use of vaccination, or a cull strategy for the associated wildlife populations.
Prognosis
Poor.
Links
Literature Search
Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation).
Mycobacteroium bovis in cattle publications
References
- Andrews, A.H, Blowey, R.W, Boyd, H and Eddy, R.G. (2004) Bovine Medicine (Second edition), Blackwell Publishing
- Blood, D.C. and Studdert, V. P. (1999) Saunders Comprehensive Veterinary Dictionary (2nd Edition) Elsevier Science
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition) Merial
- http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/tb/