Ostertagiosis - Cattle
Jump to navigation
Jump to search
Caused by Ostertagia.
Epidemiology of Ostertagiosis (Dairy Herds)
Calves are weaned early and put out to graze with other calves of similar age. Disease risk high if not controlled, and most frequently occurs when calves graze permanent pasture, especially if kept at high stocking rates.
Type 1 Disease
- Calves during their first grazing season between mid-July and October
- Disease caused by L3 ingested 3-4 weeks earlier
- High morbidity/low mortality
- Clinical signs:
- Diarrhoea
- Weight loss
- Reduced appetite
Pre-Type 2 Phase
- Calves at end of first grazing season (from October)
- Accumulation of large population (greater than 100,000) of Ostertagia EL4 (arrested stage)
- Disease caused by L3 ingested in late autumn
- Clinical signs:
- Usually none seen
Type 2 Disease
- Yearlings housed after first grazing season (February-May)
- Disease due to resumed development of waves of previously arrested EL4 ingested as L3 the previous autumn
- Low morbidity/high mortality
- Clinical signs:
- Diarrhoea
- Weight loss
- Reduced appetite
- Submandibular oedema
Epidemiology of Ostertagiosis (Beef Suckler Herd)
Calves suckle and graze with their dams. Calves are susceptible, but cows are immune. Overt disease is much less common than in dairy herds because:
Spring-Calving Herds
- Spring mortality of L3 occurs before calves eat significant amounts of grass
- Immune cows pass very few worm eggs, leading to development of very few L3
- Disease very unlikely
Autumn-Calving Herds
- Calves graze before spring mortality of L3 occurs, and will contaminate pasture
- Relatively few calves on pasture (as most of the grass is needed by the cows)
- Therefore, relatively few eggs dropped onto pasture, so disease risk higher but still low compared with dairy herds
Epidemiology of Ostertagiosis (Immunity)
- Acquired immunity slow to develop (takes whole grazing season)
- Immunity may wane during winter housing, but is rapidly re-established upon turnout
- Adult cattle solidly immune (no significant role in epidemiology of disease)
Pathogenesis
Replacement of specialised epithelia by rapidly-dividing undifferentiated cuboidal cells
- Epithelial hyperplasia (primary nodules; secondary nodules = “morocco leather”)
- Increased permeability to macromolecules (increased blood pepsinogen, hypoalbuminaemia)
Loss of Parietal (and Zymogen) cells
- Increased gastric pH → decreased protein digestion, secretion of pepsinogen and conversion of pepsinogen to pepsin; increased gastric secretion, bacterial growth/change in flora.
Diagnosis of Ostertagiosis
- Seasonal incidence
- Previous grazing history
- Clinical signs
- Faecal examination, worm egg count
- Type 1 disease: >1000 e.p.g. (eggs per gram)
- Type 2 disease: variable, often zero
- Blood pepsinogen or gastrin
- Elevated; specific indicator of abomasal damage in groups of animals
- Post mortem examination
- Fundic nodules
- Increased gastric pH
- Putrid smell
- >40,000 adult worms in lumen on mucosal surface
- Larvae in mucosa
Control of Ostertagiosis (Type 1 Disease)
- Use clean pasture (e.g. new leys, pasture not grazed by cattle previous year - but not always available)
- Delay turnout until after spring mortality of L3 (but uneconomical use of pasture/supplementary feeding)
- Dose and move to aftermath (hay/silage) in mid-July (but will not control early season disease)
If no alternate grazing available:
- Repeated anthelmintic treatment
- Monthly from mid-July (but temporary control of egg output only, cattle reinfected)
- Before mid-July on 2 or 3 occasions, e.g. ivermectin 3, 8 and 13 week treatment post-turnout (relies on residual activity of at least 2 weeks and 3 week worm prepatent period, but labour intensive)
- Intra-ruminal anthelmintic devices (minimise pasture contamination and size of autoinfection peak
- Paratect Flex (Pfizer)
- Autoworm (Schering-Plough)
- Ivomec SR bolus (Merial)
- Panacur bolus (Hoechst) (expensive)
[Note: not all of these are still on sale in the UK]
Control of Ostertagiosis (Type 2 Disease)
- Cattle exposed to low challenge at pasture in late autumn
- Unlikely to require treatment at housing
- Cattle exposed to medium/high challenge in late autumn or animals of unknown origin
- Likely to require treatment at housing using an anthelmintic active against hypobiotic larvae
- Caused by Ostertagia ostertagi.
- Economically and epidemiologically the most important gastro-intestinal parasite in the bovine in Britain.
Pathogenesis
Type I Ostertagiasis
- Ostertagia ostertagi is ingested by calves in their first year at grass.
- The parasites colonise the gastric glands of the fundus and pylorus.
- 17-21 days after ingestion, the parasites reach maturity and emerge from the gastric glands.
- Emergence in sufficient numbers causes extensive pathological changes- chronic gastritis.
- The major change is reduction in the functional gastric gland mass
- Parietal cells and zymogen cells are replaced by rapidly dividing undifferentiated, non-functional cells.
- A thickened, hyperplastic, non-functional gastric mucosa is formed.
- The major change is reduction in the functional gastric gland mass
- A non-functional gastric mucosa means that:
- Abomasal pH is raised from 2 to 7.
- Pepsinogen activation to pesin fails above pH 5.
- Proteins are not denatured.
- Bacteriostasis fails, increasing the abomasal bacterial population.
- Pepsinogen outputis reduced.
- The bowel wall becomes more permeable to macromolecules.
- The junctions between the rapidly dividing undifferentiated cells are not completely formed
- Large molecules, particularly proteins, can pass through.
- Inactivated pepsinogen passes through the incomplete junctions to the circulation, raising plasma pepsinogen levels
- Hypoalbuminaemia occurs, indicating of loss of plasma proteins into the gut lumen.
- The junctions between the rapidly dividing undifferentiated cells are not completely formed
- Abomasal pH is raised from 2 to 7.
- Impaired digestion and diarrhoea is the result of these changes, but Ostertagiais does not usually cause an acute problem.
Type II Ostertagiasis
- Ostertagia ostertagi may become hypobiotic in the Autumn.
- In heavy infestations lots of hypobiotic larvae reactivate in the Spring
- Produce severe acute gastritis (fibrinous or haemorrhagic), and even sudden death.
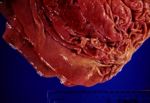
Pathology
- Lesions are typical.
- Raised hyperplastic nodules, 2-3mm in diameter with a central orifice (the opening to the parasitized gastric gland).
- In heavy infestations the nodules overlap giving the mucosa a “moroccan leather” or “crazy paving” appearance.
- Following emergence of the parasites, the surface epithelium necrotises and sloughs, and a grey-white diphtheritic membrane of protein, polymorphs and clumps of bacteria forms.