Difference between revisions of "Uraemia"
Michuang0720 (talk | contribs) |
|||
| Line 73: | Line 73: | ||
{{Learning | {{Learning | ||
| + | |Vetstream = [https://www.vetstream.com/canis/search?s=uraemia Uraemia] | ||
|flashcards = [[Veterinary Dentistry Q&A 17]] | |flashcards = [[Veterinary Dentistry Q&A 17]] | ||
|literature search = [http://www.cabdirect.org/search.html?it=any&q2=uremia&q1=uraemia&calendarInput=yyyy-mm-dd&occuring1=title&show=all&rowId=1&rowId=2&rowId=3&options1=AND&options2=OR&options3=AND&occuring3=freetext&occuring2=title&publishedend=yyyy&la=any&publishedstart=yyyy&fq=sc%3A%22ve%22&y=8&x=70 Uraemia publications] | |literature search = [http://www.cabdirect.org/search.html?it=any&q2=uremia&q1=uraemia&calendarInput=yyyy-mm-dd&occuring1=title&show=all&rowId=1&rowId=2&rowId=3&options1=AND&options2=OR&options3=AND&occuring3=freetext&occuring2=title&publishedend=yyyy&la=any&publishedstart=yyyy&fq=sc%3A%22ve%22&y=8&x=70 Uraemia publications] | ||
Revision as of 07:53, 25 June 2016
Introduction
Uraemia describes the clinical systemic syndrome that occurs in animals suffering from renal failure. Traditionally, uraemia was thought to be caused by azotaemia, an increase in the blood concentrations of urea and creatinine, but it is now apparent that multiple uraemic toxins cause derangements in many metabolic processes. Azotaemia is important in the diagnosis of uraemia but it is an insensitive indicator of renal failure, only becoming detectable when more than approximately two thirds of the nephrons are no longer functional.
Pathophysiology
The kidney has multiple metabolic functions, removing nitrogenous waste from the plasma, maintaining electrolyte and acid/base balance and producing hormones such as erythropoietin (EPO). Renal failure causes alterations in all of these functions and produces the clinical signs of uraemia.
Failure to excrete nitrogenous waste leads to azotaemia and, although this term refers specifically to urea and creatinine concentrations, multiple other forms of waste are retained and contribute to the clinical syndrome. The nitrogenous waste causes pathological changes of arteriolar fibrinoid degeneration throughout the body.
Other uraemic toxins include parathyroid hormone (PTH), phosphate, insulin, gastrin, glucagon and prolactin. PTH is released due to hypocalcaemia caused by phosphate retention and reduced production of vitamin D and its effects produce secondary renal hyperparathyroidism.
Signalment
Uraemia is described almost exclusively in animals with renal failure.
Clinical Signs
Uraemia is a complex disorder that affects multiple organ systems and produces a variety of clinical syndromes:
Gastro-intestinal Disease
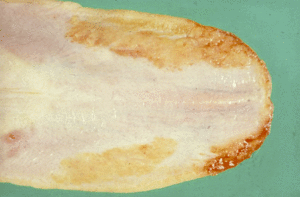
- Oral ulceration or uraemic stomatitis - This occurs especially at the fauces of the mouth and on the margins of the tongue. Uraemic vasculitis and thrombosis lead to necrosis and sloughing of the mucosa. There is irritation of the tissues from the ammonia produced by the bacterial degradation of urea. Halitosis is often a feature of this syndrome as the lesions become secondarily infected with oral bacteria such as Fusobacterium necrophorum. In severe cases, there may be extensive subepithelial necrosis and sloughing of the tip of the tongue. The lesions are often very painful and contribute to the anorexia often observed in animals with chronic kidney disease. Excessive dental calculus may be evident in animals with chronic kidney disease on oral examination.
- Gastric ulceration - This occurs for three main reasons. First, urea crosses lipid membranes freely and enters the gastro-intestinal lumen of azotaemic animals. The urea is degraded to ammonia by bacterial urease and the ammonia irritates the intestinal mucosa. This is compounded by damage to the blood vessels of the gastric submucosa by the fibrinoid necrosis that is a common feature of uraemia. Elevated serum concentrations of gastrin (which is normally metabolised in the kidney) also lead to excessive production of gastric acid from parietal cells in the stomach. Animals with gastro-duodenal ulceration may show anorexia, vomiting, haematemesis and peritonitis and haemorrhage if the ulcers perforate. Hypergastrinaemia may also cause incompetence of the pyloric sphincter of the stomach, permitting reflux of irritant bile into the stomach. Similar processes result in the development of uraemic colitis.
- Uraemic peritonitis - This is a form of chemical peritonitis that results from inflammation of the small mesothelial blood vessels.
Respiratory Disease
- Pulmonary oedema - Damage to the small vessels of the pulmonary vasculature may result in the development of vasogenic pulmonary oedema and pleural effusion with dyspnoea, tachypnoea and coughing.
- Uraemic pneumonitis - This disease occurs due to movement of leucocytes into the alveoli and alveolar septa leading to oedema.
- Can have patchy or diffuse deposition of calcium on the alveolar walls.
Electrolyte and Acid/Base Imbalances
- Electrolyte imbalances - The failure to excrete phosphate through the damaged kidneys results in hyperphosphataemia. This electrolyte complexes with calcium and also prevents the activation of vitamin D (dihydroxycholecalciferol), resulting in hypocalcaemia. This hypocalcaemia directly stimulates the production of parathyroid hormone (PTH) to try to maintain normal blood calcium levels and, in ~10% dogs with renal failure, hypercalaemia may develop due to an alteration in the set-point at which PTH is secreted. In the remaining 90%, calcium is mobilised from bone causing secondary renal hyperparathyroidism with resorption of bone, pathological fractures and fibrous osteodystrophy of the bones of skull ('rubber jaw').
- Metastatic mineralisation - The presence of excessive blood concentrations of phosphate leads to metastatic calcification in multiple tissues, particularly the rugae of the gastric mucosa, the pulmonary parenchyma and the heart.
- Metabolic acidosis - The kidney plays a vital role in the regulation of the normal acid/base balance.
- Parathyroid hyperplasia
Cardiovascular Disease
- Atrial rupture - Mineralisation of the atria reduces their normal compliance and renders them susceptible to rupture. The resultant haemopericardium is often fatal as it causes acute cardiac tamponade.
- Arrhthymias
- Necrotic endocarditis - necrosis of the endocardium in the left atrium. Lesions may also occur in the pulmonary artery and aorta with erosions being associated with thrombus formation.
- Cardiac hypertrophy, especially left-sided, as a result of hypertension following release of renin from chronically damaged kidneys.
Haematological Disease
- Thrombocytopathia - The reduction in platelet function occur due to multiple alterations in normal thrombocyte metabolism.
- Anaemia - This occurs because the diseased kidneys produce less erythropoietin than normal and the uraemic toxins (especially PTH) decrease the lifespan of existing red blood cells. Erythrocytes may also be damaged as they pass along inflamed vessel walls, a form of microangiopathic haemolysis. Chronic gastro-intestinal haemorrhage may also result in iron deficiency.
Neuromuscular Disease
- Neurological disease - The presence of extremely high concentrations of urea and creatinine may induce reduced consciousness or uraemic seizures and this is usually a terminal event. PTH has also been implicated in the development of uraemic encephalopathy, possibly due to alterations in calcium pumps. Animals may enter a so-called twitch-convulsive state where they suffer concurrent tremors, myoclonus and seizures. Peripheral neuropathies may contribute to the reduced hindlimb function that is commonly ascribed to hypokalaemic myopathy.
- Myopathy - Hypokalaemic animals with chronic kidney disease may suffer severe muscle weakness with a plantigrade stance and cervical ventroflexion in cats which lack a nuchal ligament.
Laboratory Tests
Serum biochemistry will show elevated levels of urea and creatinine. Animals with acute renal failure may by hyperkalaemic and hyperphosphataemic but cats with chronic renal failure are often hypokalaemic, hyperphosphataemic and hyponatraemic. Blood ionised calcium concentration may be reduced (in ~90% cases) or elevated (in ~10% cases).
In cases of chronic renal failure, there may be a normocytic, normochromic, non-regenerative anaemia. Platelet levels are usually normal despite any reduction in function.
Other Tests
Examination of a urine sample may show signs consistent with renal failure. Animals with acute renal failure may be anuric and those with chronic renal failure may be polyuric but in both cases, the urine will be isosthenuric (with a specific gravity of 1.008-1.012).
Diagnostic Imaging
Plain radiographs may show the presence of metastatic calcification in multiple organs, including the rugae of the gastric mucosa, the heart base and atria and the pulmonary parenchyma. Renomegaly or an irregular outline may be apparent in cases with acute or chronic renal failure, respectively.
Animals with secondary renal hyperparathyroidism may show resorption of bone from the skull (particularly the alveolar bone and lamina dura around the teeth) and the terminal phalanges.
Ultrasound scans of the kidney may show changes in size and shape, the presence of pseudocysts or dilated renal pelvises or other disruptions to the renal parenchyma.
Treatment
The underlying cause of the renal failure should be treated. To alleviate the clinical signs of uraemia, it is particularly important to restrict phosphate by feeding a low phosphate diet and using phosphate-binding drugs such as aluminium hydroxide or chitosan. Deficient electroylytes should be supplemented but calcium should only be administered after hyperphosphataemia has been corrected to prevent further mineralisation of soft tissues.
Various gastro-protectant drugs can be prescribed to manage the gastro-intestinal signs of uraemia, including sucralfate and the acid secretory inhibitors ranitidine, cimetidine and omeprazole. Appetite stimulants such as mirtazapine can also be administered to anorexic animals to encourage voluntary food intake. Oral pain can be controlled by topical application of lidocaine gel or sucralfate paste.
A major advance in the management of uraemia has been the introduction of recombinant erythropoietin which can be administered to anaemic animals as a series of subcutaneous injections. Some animals may become resistant to its effects over time due to the development of an immune response to the recombinant protein.
Prognosis
Renal failure that is sufficiently severe to cause uraemia is very severe and carries a poor prognosis for recovery. Lost renal function cannot be recovered and the best that can be hoped for is to manage the clinical syndrome as long as the patient maintains an acceptable quality of life.
| Uraemia Learning Resources | |
|---|---|
To reach the Vetstream content, please select |
Canis, Felis, Lapis or Equis |
 Test your knowledge using flashcard type questions |
Veterinary Dentistry Q&A 17 |
 Search for recent publications via CAB Abstract (CABI log in required) |
Uraemia publications |
References
Ettinger, S.J, Feldman, E.C. (2005) Textbook of Veterinary Internal Medicine (6th edition, volume 2) Elsevier Saunders
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
Error in widget FBRecommend: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt697c18c20bfad6_33586573 Error in widget google+: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt697c18c22a6b57_10971093 Error in widget TwitterTweet: unable to write file /var/www/wikivet.net/extensions/Widgets/compiled_templates/wrt697c18c243c009_59940097
|
| WikiVet® Introduction - Help WikiVet - Report a Problem |