Anticoagulant Rodenticide Toxicity
| This article is still under construction. |
Also known as: warfarin toxicity/poisoning, anticoagulant rodenticide poisoning, vitamin K antagonist toxicity/poisoning.
Description
Anticoagulant rodenticides were first discovered during ingvestigations into mouldy sweet clover poisoning in cattle1. In this condition, naturally occuring coumarin in clover is converted by fungi to a toxic agent, dicumarol, which causes a haemorrhagic syndrome when ingested. Initially, warfarin was synthesised and used in this way for rodent control, but as rodents have developed a resistance to the substance new, second generation anticoagulant rodenticides have been developed. These include coumarin (bromadiolone and brodifacoum) and indandione (pindone and diaphacinone) rodenticides, which along with warfarin may cause toxicity following accidental ingestion or malicious administration in animals.
Anticoagulant rodenticide toxiticy is one of the most common causes of acquired coagulopathy in small animals. Warfarin itself has a short half-life and a fairly low toxicity in non-rodent species, so unless large or repeated doses are consumed clinical bleeding is rare. However, the second generation anticoagulant rodenticides are far more potent, with tendency to accumulate in the liver and a long half life (4-6 days) owing to high levels of plasma protein binding2, 3. These newer drugs are therefore more commonly implicated in cases of poisoning3, and it is possible for a domestic animal to acquire secondary poisoning by ingesting a killed rodent2. High plasma protein binding also means that the effects of anticoagulant rodenticides are potentiated by administration of other highly plasma protein bound drugs, and low plasma albumin levels.
Mechanism of Toxicity
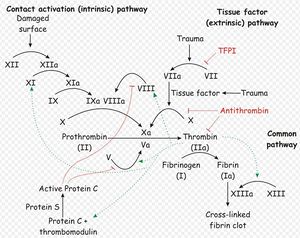
Normally, haemostastis is maintained by three key events3. Firstly, platelets are activated, adhere to endothelial connective tissue and aggregate to form a platelet plug. Next, substances are released that trigger coagulation and vasoconstriction. Finally, fibrinogen is polymerised to fibrin which reinforces the platelet plug. Some components of the coagulation and fibrin formation stages are dependent on vitamin K, and it is these which are influenced by anticoagulant rodenticide activity.
Two simultaneous cascades are activated to achieve coagulation: the intrinsic and extrinsic pathways. The intrinsic pathway is activated by contact with collagen due to blood vessel injury and involves the clotting factors XII, XI, IX and VIII. The extrinsic pathway is triggered by tissue injury and involves the factor VII. These pathways progress independently before converging at the common pathway, which involves the factors X, V, II and I and ultimately results in the formation of fibrin from fibrinogen.
Within each of the three arms of the coagulation cascade, certain clotting factors are dependent on vitamin K for activity. These include factor VII, factor XI and factors II and X in the extrinsic, intrinsic and common pathways respectively. Vitamin K carboxylates these factors to their fuctional forms, and in the process itself becomes oxidised. Vitamin K is always required for the production of new II, VII, IX, and X in the liver and levels are tightly regulated. It is therefore essential that vitamin K is recycled after it is oxidised in the carboxylation reaction, and the enzyme vitamin K epoxide reductase is respsonsible for this.
Anticoagulant rodenticides competitively inhibit vitamin K epoxide reductase4, preventing the recyling of vitamin K and depriving the liver of the active, reduced form of the vitamin1-6. Activation of factors II, VII, IX and X ceases, but there is a quantity of these already in the circulation that are not affected. A time-lag therefore exists between ingestion of anticoagulant rodenticide and the clinical manifestation of toxicity (unchecked haemorrhage), while the supply of still-viable, vitamin K-dependent clotting factors reach the end of their life span. This delay is around 5 days in length3, and may mean that patients present late to veterinary practices after intoxication6.
Since factor VII has a half-life of only 6 hours, the extrinsic pathway is the first to be affected. This causes slight impairement of haemostasis is impaired slightly giving a mild degree of haemorrhage, but the intrinsic pathway is still functional and is able to prevent the development of overt clinical signs. After around 14 hours, factor IX of the intrinsic pathway reaches the end of its life-span, and this pathway ceases to operate. Haemorrhage can then proceed unchecked, and clinical signs become obvious. Coumarin and indandosides txicity may also increase the fragility of blood vessels, exacerbating the problem by causing bleeding at sites that are not subject to trauma6.
Similar Conditions
Malabsorption syndromes and sterilisation of the gastrointestinal tract by prolonged antibiotic usage will also result in the depletion of vitamin K-dependent clotting factors7. In herbivores, fungi growing on poorly prepared hay or silage containing sweet vernal grass or sweet clover may break down natural coumarins in the plants to form dicoumarol and cause poisoning. in herbivores.
Signalment
Anticoagulant rodenticide toxcity is most often seen in dogs, due to their scavenging behaviour and the fact they appear to find rodent bait especially palatable. Farm dogs are particularly at risk since rodenticides are frequently used in this environment and many dogs are allowed to roam freely outdoors. In the cat, toxicity usually occurs via the consumption of poisoned rodents. Anticoagulant rodenticide toxicity has also been reported in the pig, and also in barn owls who have consumed rodents poisoned with second generation anticoagulant rodenticides6.
Diagnosis
Ideally, a diagnosis of anticoagulant rodenticide toxicosis should be made based on a history of ingestion of the substance. Failing this, clinical signs, certain laboratory parameters and response to treatment will be suggestive of the condition.
Clinical Signs
As described above, the onset of clinical signs in anticoagulant rodenticide toxicosis is delayed for up to five days while previously formed clotting factors reach the end of their life-spans and function facts become depleted. is delayed for several days post-exposure while the plasma concentrations of the vitamin K-dependent clotting factors become depleted. Symptoms may be non-specific if there is internal bleeding, and might include depression, weakness, pallor, dyspnoea, abdominal swelling, or even sudden death. Other possible signs include anaemia, external haematomas, bruising, excessive bleeding from venepuncture sites or other sites of injury, epistaxis, haematemesis, haematochezia, melaena, haematuria and/or lameness.
Clinical signs generally reflect some manifestation of hemorrhage, including anemia, hematomas, melena, hemothorax, hyphema, epistaxis, hemoptysis, and hematuria. Signs dependent on hemorrhage, such as weakness, ataxia, colic, and polypnea, may be seen. Depression and anorexia occur in all species even before bleeding occurs.
differentials Other causes of blood loss and anaemia: Trauma and clotting defects such as inherited conditions, autoimmune disorders, chronic liver disease and disseminated intravascular coagulation (DIC).
- Other causes of dyspnoea: Thoracic fluid, heart disease, lung disease and
respiratory obstruction.
- Other causes of acute collapse: Trauma, endotoxaemia and causes of shock
Laboratory Tests
Coagulation screening tests are unlikely to reveal abnormalities until at least 36 to 72 hours post-exposure. The prothrombin time (PT) generally becomes prolonged first (by 36 to 48 hours), since F-VII, a component of the tissue factor-mediated coagulation pathway, has the shortest half-life (about six hours) and is therefore the first factor to become depleted. The partial thromboplastin time (PTT) and activated clotting time (ACT) are usually prolonged by 48 to 72 hours post-exposure. The thrombin clotting time (TCT), platelet count and buccal mucosal bleeding time (BMBT) (an assessment of platelet function) are usually normal (see table below). The so-called 'proteins induced by vitamin K antagonism' (PIVKA) are acarboxylated proteins formed as a result of anticoagulant rodenticide toxicity. While not normally detected in the circulation, these increase in the plasma of poisoned animals and can be detected using the PIVKA test which is available through some veterinary diagnostic laboratories. PIVKA are usually cleared within 12 hours of administration of vitamin K. Samples for coagulation testing should be collected before initiating vitamin K therapy. Other possible confirmatory tests include quantitation of vitamin K epoxide concentrations and determination of the specific anticoagulant in the blood, liver and/or stomach contents.
Pathology
- Gastric haemorrhage
- Haemorrhage elsewhere in body, particularly mediastinum
Treatment
Treatment of anticoagulant rodenticide poisoning must be supportive in nature and is directed at correcting the hypovolaemia and coagulopathy. Fresh blood or plasma will help to correct the hypovolaemia and enhance haemostasis by restoring depleted clotting factors. Vitamin K1 (5 mg/kg) should be given as a loading dose subcutaneously at multiple sites, followed by subcutaneous or oral doses (1.25 to 2.5 mg/kg) at eight to 12 hour intervals for as long as necessary (until the toxin is metabolised or excreted). The duration of treatment will depend on the anticoagulant involved. A one-week treatment may be undertaken initially. The PT and PTT must be checked 48 to 72 hours after cessation of vitamin K1 therapy. With the more persistent anticoagulants, these clotting tests may become prolonged again, indicating a residual toxic effect and the need for continued vitamin K1 therapy. In some patients, treatment for a month or more may be required. Although less expensive, vitamin K3 is relatively ineffective and is not recommended as a treatment for anticoagulant rodenticide toxicity. Hypocoagulable patients are at great risk of internal haemorrhage. Physical activity must therefore be minimised and their condition monitored closely. Other forms of supportive therapy may be indicated to reduce discomfort and to protect the animal from injury. The administration of drugs with known antiplatelet effects is contraindicated, as is the administration of agents by intramuscular injection.
If ingestion was recent (in past three hours) induce vomiting. Stomach lavage may also be indicated if dogs fail to vomit. Coumarin rodenticide preparations are often in the form of blue or green granules.
- Give the specific antidote - vitamin K. Phytomenadione, a vitamin K1 analogue available as tablets or injection
(Konakion; Roche), is the drug of choice and reverses low prothrombin levels in 30 minutes. Menadiol (Synkavit; Roche) is a synthetic K3 and is not as effective. Dose. 2 - 5 to 10 mg three times daily orally for five days because most coumarins are metabolised and excreted slowly over two to four days, and longer in some instances. If clinical signs are severe can give 5 mg intravenously over six to eight hours. However, as anaphalactic reactions to intravenous administration have been reported in the dog intramuscular route is preferable.
- Give a whole blood transfusion - this replaces the clotting factors as well as replacing blood loss through haemorrhage.
Vitamin K1 is antidotal. Recommended dosages vary from 0.25-2.5 mg/kg in warfarin (coumarin) exposure, to 2.5-5 mg/kg in the case of long-acting rodenticide intoxication (diphacinone, brodifacoum, bromadiolone). Vitamin K1 is administered SC (with the smallest possible needle to minimize hemorrhage) in several locations to speed absorption. IV administration of vitamin K1 is contraindicated, as anaphylaxis may occasionally result. The oral form of K1 may be used daily after the first day, commonly at the same level as the loading dose (divided bid). Fresh or frozen plasma (9 mL/kg) or whole blood (20 mL/kg) IV is required to replace needed clotting factors and RBC if bleeding is severe. One week of vitamin K1 treatment is usually sufficient for first-generation anticoagulants. For intermediate and second-generation anticoagulants or if anticoagulant type is unknown, treatment should continue for 2-4 wk to control longterm effects. Administration of oral vitamin K1 with a fat-containing ration, such as canned dog food, increases its bioavailability 4-5 times as compared with vitamin K1 given PO alone. Coagulation should be monitored weekly until values remain normal for 5-6 days after cessation of therapy. Vitamin K3 given as a feed supplement is ineffective in the treatment of anticoagulant rodenticide toxicosis. Additional supportive therapy may be indicated, including thoracocentesis (to relieve dyspnea due to hemothorax) and supplemental oxygen if needed.
Prognosis
Links
References
- Murphy, M J and Talcott, P A (2005) Anticoagulant Rodenticides. In Small Animal Toxicology (Second Edition), Saunders.
- Campbell, A (1999) Common causes of poisoning in small animals. In Practice, 21(5), 244-249.
- Beasley, V (1999) Toxicants that Interfere with the Function of Vitamin K. In Veterinary Toxicology, International Veterinary Information Service.
- Mayer, S (1990) Coumarin Derivatives. In Practice, 12(4), 174-175.
- Johnstone, I (2002) Bleeding disorders in dogs 2. Acquired disorders. In Practice, 24(2), 62-68.
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Dodds, W J (2005) Bleeding Disorders in Animals. In Proceedings of the World Small Animal Veterinary Association 2005, IVIS.
- DeWilde, L (2007) Why is Fluffy Bleeding? Secondary Hemostatic Disorders. In Proceedings of the North American Veterinary Conference 2007, NAVC.
- Keen, P and Livingston, A (1983) Adverse reactions to drugs. In Practice, 5(5), 174-180.