Fasciolosis
| This article is still under construction. |
| Also known as: | Fascioliasis Fasciolasis Fluke |
Description
Fasciolosis is a condition of ruminants which causes significant economic losses through death, subclinical and clinical disease. The causative organism is the trematode Fasciola hepatica which primarily parasitises the bile ducts of sheep and cattle but may occasionally be found in the horse. Lymnaea truncatula, a mud snail, is the intermediate host of Fasciola hepatica, and transmission of disease is dependent on the presence of appropriate snail habitats. These habitats are more plentiful in areas of high rainfall, such as the western British Isles. However, infected animals may be found outwith these areas due to the transportation of livestock. The association of fasciolosis with wetter habitats lends a seasonal nature to disease outbreaks, and can help predict the severity of these.
Geographical distribution of disease
Transmission is dependent on the snail, and therefore associated with snail habitats.
Weather patterns and disease risk
Wet summer
Many fluke and snail eggs hatching and snail habitats expanded, therefore:
→ snail population increases rapidly
→ many infected snails
→ many cercariae shed
→ high density of metacercariae on herbage
→ increased risk of disease
Dry summer Few fluke and snail eggs hatching and snails restricted to permanent habitats, therefore:
→ snail populations small and overcrowded
→ few infected snails, high mortality
→ few cercariae shed
→ few metacercariae confined to restricted areas
→ reduced risk of disease
- Large numbers of metacercariae ingested over a short period of time leads to acute diseasegnosis==
- Smaller numbers ingested over a longer period of time leads to chronic disease
- Feed intake
- Fluke infections cause reduced food intake
NOTE: chronic fasciolosis occurs at a time of year when animals are on a low plane of nutrition. This combined with the reduced food intake causes a significant effect on the development and severity of clinical and subclinical fasciolosis.
- Species susceptibility
- The proportion of flukes that reach the bile ducts is determined mainly by
- The fibroplastic potential of the liver
- The effectiveness of the protective immune responses (which are ineffective in sheep)
- Therefore, establishment rate is sheep>cattle>pig
- The proportion of flukes that reach the bile ducts is determined mainly by
A similar but slightly larger species, F. gigantica, occurs in wetter tropical regions.
Signalment
Diagnosis
Clinical Signs
Ovine Fasciolosis
Acute fasciolosis
- Sudden death
- Normally September-November
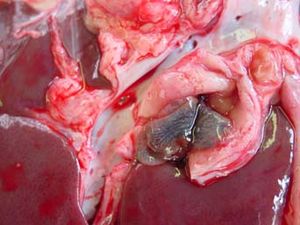
- Enlarged pale, friable, haemorrhagic liver
- More than 1000 immature flukes in liver parenchyma
NOTE: Acute disease is rarely seen in cattle
Sub-acute fasciolosis
- Rapid weight-loss over 1-2 weeks
- Occurs October-December
- Normochromic anaemia
- Enlarged liver with large haemorrhages
- More than 500 flukes - 50:50 immature and adult
Chronic fasciolosis
- Progressive weight-loss over weeks or months
- Occurs January-March
- Normochromic anaemia often becoming hypochromic
- Hypoalbuminaemia leading to oedema
- Small, distorted cirrhotic liver
- Enlarged bile ducts
- More than 250 adult flukes
NOTE: Calcification of bile duct in cattle → 'pipe-stem' liver
Sub-clinical effects
- In sheep, fleece weight and fibre quality are affected even by small fluke burdens. There is some evidence that reproductive performance (number of lambs born and growth-rate of lambs) is inversely influenced, but this has not been well documented.
- Liver condemnations cause economic losses at slaughter
- Longer finishing times to slaughter weight and reduced carcass value in beef cattle
- Reduced milk yield and quality in dairy cows
Metacercariae excyst → immature flukes present in the small intestine → migrate across the peritoneal cavity (about 1 week) → to the liver → migrate through the liver parenchyma for 6-7 weeks becoming more destructive as they grow → enter bile ducts
- The prepatent period is 10-12 weeks
Acute Fascioliasis
The acute disease is a less common type of Fasciola hepatica, and generally occurs 2-6 weeks after large ingestion of metacercariae. The young liver flukes migrate through the liver parenchyma causing severe haemorrhaging, due to the damage to the liver vasculature.
This occurs in late autumn and winter, mainly between the months of August to October. Outbreaks of acute fascioliasis usually present as sudden deaths. On examination infected animals are weak, with pale mucous membranes. They may also have enlarged livers, and the liver surface may be cover with a fibrinous peritonitis, particularly evident on the ventral lobe. Tracts become filled with blood and degenerate hepatocytes later infiltrated with eosinophils, lymphocytes and replaced by fibrosis.
Subactute Fascioliasis
This is caused by ingestion of metacercariae over a longer period of time. Some may have migrated to the bile ducts, causing cholangitis, whilst other metacercariae are migrating through the liver causing lesions similar to those present in acute fascioliasis. The infected host may present with severe haemorrhagic anaemia, with hypoalbuminaemia, rapid loss of body condition, reduced appetite, pale mucous membranes, and submandibular oedema may also be present. On post-mortem, an enlarged liver is common and haemorrhagic tracts are usually visible on the liver surface. If left untreated, it is often fatal. This form of fascioliasis occurs around 6-10 weeks after ingestion of the metacercariae by the host, and like acute fascioliasis occurs in late autumn and winter.
Chronic Fascioliasis
This is usually seen in late winter, early spring and is currently the most common fascoloiasis seen. It occurs around 4-5 months after ingestion of the metacercariae. Hypochromic and macrocytic anaemia and hypoalbuminaemia are common, as the adult flukes are capable of sucking up to 0.5ml of blood each day. In heavy infections, this can prove to be a severe loss.
Infected animals may present with progressive loss of body condtion, reduced appeptite, which along with hypoalbuminaemia can result in an gaunt animal. Other common signs include pale mucous membranes, and submandibular oedema, more commonly known as 'bottle jaw.' On biopsy the liver will have an irregular shape, distorted shape with areas of fibrous tissue replacing the cells damaged by the migrating flukes. The bile ducts appear dilated, and dark, and it is often possible to express numerous numbers of adult flukes from within the ducts. Pathology is similar in both sheep and cattle, expect in cattle you may see calcification of the bile ducts, and enlargement of the gall bladder. The calcified bile ducts are often seen protruding from the liver surface, which is known as 'pipe stem liver.'
Laboratory Tests
This is performed primarily on the clinical findings, seasonal occurance, as well as a previous history of fasciolosis on the farm. These along with post-mortem form the basis for diagnosis of Fasciola hepatica. In practice, diagnosis of ovine fasciolosis is often more straightforward than bovine fasciolosis.
Examination of faeces for liver fluke eggs is also useful, and may be complemented by laboratory tests. Firstly the measurement of Glutamate dehydrogenase (GLDH) is used. This is an enzyme released by damaged hepatic cells, and levels become elevated within the first few weeks of infection. Another test commonly used is measuring the glutamyl transpeptidase (GGT), and this indicates damage to the epithelial cells of the bile ducts, and is seen later in the cycle, and maintained for a longer duration.
An ELISA can also be used in the detection process. It identifies antibodies against flukes in the serum and milk samples, and is deemd to be extremely reliable in this case.
Acute fasciolosis
- Clincal history
- Season
- Grazing
- Post-mortem examination
Chronic fasciolosis
- Clinical history
- Season
- Grazing
- Lethargy
- Weight loss
- Anaemia (pallor)
- Hypoalbuminaemia (bottle-jaw)
- Gamma-glutamyl transpeptidase increase (i.e. increase in hepatic enzymes)
- Fluke eggs in faeces
Pathology
Pathogenesis of acute fasciolosis
- Liver pathology
- Flukes develop from 0.1mm-1cm within the liver parenchyma causing trauma, necrotic tracts, and haemorrhages
- Glutamate dehydrogenase (GDH) is released by damaged cells
- Acute damage to liver causes post-necrotic scarring → shrinkage of affected tissues and hypertrophy of normal tissue → the typical appearance of the liver in chronic disease
- Chronic damage to bile ducts → peribiliary fibrosis
(Note: other complex events also occur, including disruption of haemodynamics, monolobular fibrosis, egg-granulomas etc.)
- Black disease (Infectious Necrotic Hepatitis)
- caused by a toxin produced by Clostridium novyi type B.
- It is commonly associated with liver fluke infestation because migrating flukes → liver necrosis → anaerobic conditions → clostridial multiplication → toxin production → disease
Pathogenesis of chronic fasciolosis
- Bile duct damage
- Adult flukes (2-5cm long) in bile ducts feed on epithelium and blood
- Chronic inflammatory responses → fibrosis of bile duct wall (and, in cattle, calcification)
- Gamma glutamyl transpeptidase released by damaged cells
- Ulceration and haemorrhage of bile duct → epithelial hyperplasia and increased mucosal permeability
- Anaemia
- 250 flukes → up to 50ml of blood loss daily → 10 times increase in rate of erythropoiesis → normochronic anaemia until iron stores are exhausted → hypochromic anaemia.
- Hypoalbuminaemia
- Albumin (and other plasma proteins) lost into bile duct because of
- Whole blood loss
- Increased epithelial permeability
- → Increased catabolic rate by 2.5x → increased nitrogen loss via urine
- The effects seen depend on the magnitude of nitrogen loss
- There is no obvious effect (although animal is still in abnormal physiological state)
- Reduced weight-gain and/or wool growth and/or milk production
- Loss of body tissue (i.e. weight-loss); hypoalbuminaemia → reduced plasma oncotic pressure → oedema
Treatment
Forecasting Fasciolosis
Several prediction models have been developed. They evaluate the wetness of the soil from May to October by taking account of rainfall patterns and evapo-transpiration. Seasonal weighting factors are applied. June is a particularly influential month.
A drought in late summer can reverse a potentially dangerous trend, and so unqualified forecasts should not be issued prematurely.
A high snail density will only lead to disease if infection is present (i.e. if fluke eggs have been deposited onto the habitat by farm or wild animals). Local biological interpretation of computer generated predictions is therefore required.
These forecasting models can provide valuable information for the farming community. MAFF (before DEFRA) used to provide an annual forecast, but this is now discontinued.
Control
Anthelmintics
- Few flukicides kill all parasitic developmental stages
- Not all products, therefore, are suitable for controlling acute outbreaks
- The anthelmintic with the broadest spectrum of activity against immature and adult F. hepatica is Triclabendazole
- Triclabendazole-resistant F. hepatica populations are beginning to emerge
- The more potent products tend to be the most expensive
- Many flukicides bind to plasma protein and have long plasma half-lives
Chemoprophylaxis
There are several control objectives
- To prevent fluke eggs being dropped onto snail habitats
- This is done by treating sheep/cattle with an adulticidal drug in the late winter/early spring
- To protect animals grazing pasture known to be contaminated with metacercariae. The choice of drug, time of treatment and dosing interval will depend on
- Whether you are trying to prevent acute or chronic disease
- The likely intensity of challenge (local knowledge/fluke forecast)
- The persistent effect of the drug (i.e. the period after dosing, during which the animal is protected from reinfection)
Vaccination
- A recombinant vaccine providing approximately 70% protection for cattle is being developed
- It exerts its effect by stimulating a range of immune responses not normally seen in chronically infected animals (including TH1-type responses)
Molluscicides
- These have been employed with success in the past, but are no longer used. This is because they have to be applied before any fluke forecast can be issued. (Farmers are unwilling to invest in control measures before they are known to be necessary). Also, they have to be applied very carefully as snails can rapidly recolonise sprayed land if any habitat has been missed
Alternative strategies
- An ability to recognise and define the extent of snail habitats allows alternative cost-effective control options such as fencing and drainage
Treatment
If the fuke is present treat with triclabendazole, which is effective against all stages of Fasciola hepatica. Treatment should be applied in September/October and again in January, if faecal egg count is still postitive. One may also treat against adult only stages in May/June, preventing any future pasture contamination. However, do not use the same treatment in September/October as used in May/June, as resistance to drugs is becoming a real problem within the UK due to overuse. If it has been a particularly wet season, it may be necessary to treat again, as Fasciola hepatica becomes more prevalent under such conditions.
Isolation and treatment of all new animals entering from another farm has also be shown to be effective. Other control measures include fencing off wet areas, and increasing soil drainage.
Prognosis
References
Links
- The causative organism, Fasciola hepatica.