| This article is still under construction. |
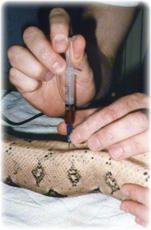
The sites for obtaining blood samples in snakes include the ventral tail vein, the palatine vein and cardiocentesis. Restraint is by gentle, careful handling or by chemical means. Asepsis is extremely important during blood collection and the site of collection should be prepared as if for surgery. Prior to collection it is important to have all materials and equipment ready: cotton wool, antiseptic (chlorhexidine or a povidone-iodine detergent), needle and syringe of suitable sizes, and the blood handling materials.
Ventral tail vein
Blood may be taken from the ventral tail vein in physically restrained snakes. Sedation may be necessary though.
- The ventral tail vein is located in the midline ventral to the coccygeal vertebrae.
- Needle size - 0.5-0.8 mm (25-21g) x 16-25 mm (5/8-1') usually.
- The needle is directed at 45-60 degrees craniodorsally between caudal scales in the ventral tail midline. Avoid the hemipenes of males.
- While maintaining slight negative pressure the needle is advanced until blood is observed or contact is made with the ventral surface of a vertebra.
- If not successful withdraw slightly or redirect the needle so that it is definitely in the midline.
Palatine vein
The dorsal palatine vein courses longitudinally along the ventromedial surface of the palate and small amounts of blood are obtainable in well-restrained unsedated snakes.
- The palatine veins are located on the ventral aspect of the snake’s hard palate.
- Needle size - 0.5 mm (25g) x 16 mm (5/8').
- With the mouth opened the palatine veins can be visualised.
- Care is necessary in restraint and haemorrhage must be considered. This can be controlled by applying pressure with a cotton-tipped applicator that has been adrenalinised.
Cardiocentesis
Cardiocentesis is an atraumatic procedure under sedation that gives sufficient amounts of blood for analysis.
- The snake is restrained in dorsal recumbency and the apex beat of the heart is located by observation or Doppler.
- Needle size - 0.5-0.6 mm (25-23g) x 16-25 mm (5/8-1') usually.
- The needle is directed into the apex of the beating ventricle. The heart can be immobilised between the thumb and forefinger and the needle is advanced at 45 degrees in a craniodorsal direction or the needle can be directed at 90 degrees in a dorsal direction.
- Blood enters with each heartbeat.
- Pericardial fluid has been obtained if only clear fluid is withdrawn.
Amount
In reptiles the total blood volume varies with species but is approximately 5-8% bodyweight. The maximum that can be drawn safely is 10% of the total blood volume. A healthy 100g reptile can therefore have 0.5ml safely taken. Weigh accurately and make the calculations before blood is withdrawn! Though microtechniques for biochemistry are available in some laboratories, it is generally advisable to take 1.5ml of blood for complete haematology and biochemistry. Check with your laboratory about handling if you are submitting less than this amount.
Protocol for blood handling
The following protocol is for reptiles that weigh over 300 g. For reptiles under this weight less plasma will be available for biochemistry. It may be necessary to prioritise your tests.
- Essential materials and equipment - 0.5 ml heparin tube (orange), 1.0 ml heparin and gel tube (green), 4 coverslips (or 3 microscope slides).
- Advisable - microhaematocrit tube, centrifuge and refractometer.
- Take blood aseptically. 1.0 ml of blood is put in the heparin/gel tube for biochemistry and 0.5 ml into the heparin tube for haematology.
- Two blood films are made immediately the needle is withdrawn from the vein. Slide smears are adequate but coverslip smears are superior.
- A haematocrit-capillary tube is filled for centrifuging for PCV then total protein by refractometer. This step is not essential but will give immediate results. A buffy coat smear can also be obtained.
- The sample for biochemistry is centrifuged immediately to separate the plasma. A delay may cause artificial changes to the biochemical parameters (e.g. potassium concentration declines due a shift of ions from the plasma into the red blood cells).
Use of anticoagulants
EDTA may be suitable for some species but, as a general rule, use lithium heparin for haematology since EDTA lyses erythrocytes of some species of reptiles. Blood films are best made with fresh blood without an anticoagulant immediately following collection and then air-dried. Heparin creates a blue tinge to blood films and causes clumping of the thrombocytes and leukocytes whereas those made from blood without anticoagulant give superior cellular morphology.