Also known as: TSE – Transmissible spongiform encephalopathy, Paraplexia enzootica ovium
Introduction
Scrapie is a progressive, fatal and non-febrile neurological disorder affecting sheep and goats. It belongs to a group of diseases called transmissible spongiform encephalopathy (TSE) and other TSE’s include Creutzfeldt-Jakob disease in humans, BSE, chronic wasting disease (CWD) in elk and deer, transmissible mink encephalopathy and feline spongiform encephalopathy has been found within cats in the UK. The disease is believed to be caused by a conformational change in the prion (PrP). A prion is a protein that occurs normally in the nervous and lymphoreticular tissues. It is only when the prion changes conformation into a protease-resistant protein PrPsc that it causes degeneration of neurological tissue. The disease causes astrocyte proliferation and then vacuolization of neurons but demyelination does not occur [1]. The abnormal protein is thought to act as a catalyst to convert more of the host’s protein into this abnormal form. The disease has been notifiable in the EU since 1993 but unlike BSE there is no evidence to suggest that scrapie is a risk to human health [2], [3],[4], [5]. Studies have suggested that after ingestion, PrPsc first accumulates in Peyer’s patches of the small intestine, gut-associated lymphoid tissues (GALT) and ganglia of the enteric nervous system [6],[7], [8], [9], [10], it then spreads throughout the lymph nodes, tonsils, spleen, and into the peripheral nervous tissue. It finally found in the brain several months later. The disease is thought to have come from imported Merino sheep from Spain and has since spread through the movement of infected sheep. Only Australia and New Zealand are recognized as being currently free of scrapie.
Signalment
Scrapie affects the majority of sheep between 3 and 5 years of age and has a long incubation period of two to five years. It is extremely durable and is able to withstand high temepratures and concentrations of formaldehyde. Unlike BSE, scrapie is influenced by breed and genetic variation of the PrP gene within sheep populations, which can affect the infectivity and incubation period of the scrapie. The disease has been shown to be effectively transmitted during lambing [11], [12], and experimental studies have shown that the ingestion of infected placenta can spread the disease in sheep and goats [13].
Clinical Signs
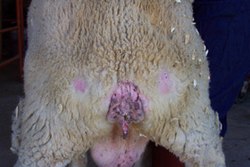
Early clinical signs may include subtle behavioural and neuorological changes. Sheep often have a fixed gaze, and suffer from bruxism, fine tremors, and hyperaesthesia to sound or sudden movements. Affected animals may later become exercise intolerant and develop progressive ataxia. Sheep often find difficulty in turning, sway on their hind hindquarters and have gait abnormalities such as a high stepping gait in the forelimbs or a bunny hopping gait in the hindlimbs. Some sheep have intense pruritis that leads to compulsive rubbing, nibbling at the skin, or scraping against fixed objects. Wool loss is typically seen over the hindquarters and lateral thorax. Lip smacking or nibbling reflex can often be elicited by scratching over the lumbar region, which is characteristic og scrapie. Significant weight loss with or without a decrease in appetite, weakness, recumbency, and death are all seen within the later stages of the disease.
Diagnosis
A pre-emptive diagnosis of scrapie may be made from the above clinical signs and history. There are no serological test available for scrapie, as is does not evoke an immune or inflammatory response. Diagnosis is confirmed on postmortem and PrPSc can be isolated from brainstem or lymphoid tissues by Western immunoblot, immunohistochemistry (IHC) and elisa tests. Immunohistochemistry usually shows vacuolation and an accumulation of prion proteins in various parts of the CNS (medulla, pons, midbrain, and spinal cord). However vacuolation is not completely diagnostic since it may also be present to a lesser extent in the brains of healthy sheep [14], [15]. In most instances the abnormal prion is resistant to protein kinase digestion, a feature used in diagnostic techniques. IHC staining of tonsil and lymphoid biopsies have been used for preclinical scrapie testing.
Differential diagnosis: Viral encephalomyelitides (pseudorabies or Aujeszky’s disease, rabies, maedi visna), Bacterial meningoencephalomyelitides (listeriosis), Pregnancy toxemia (ketosis), Hypocalcemis-hypomagnesemia, Toxins (mercury, lead, organophosphates, plant toxins) and Mange, lice, bacterial dermatitis [16].
Treatment
Scrapie is a fatal condition and no effective treatment is currently available
Control
Good husbandry and hygiene around lambing can greatly reduce the infectious load. It is recommended that individual straw bale pens are used which can be destroyed after each lambing and that contaminated bedding and placenta should be removed immediately. Infection can be minimised by maintaining a closed flock and only obtaining replacement ewes or breeding rams from scrapie-free flocks. Animals of resistant genotypes should be used for breeding to further minimize the risk of scrapie infection in a flock [17], [18], [19], [20]. Genetic resistance to scrapie depends on the prion genotype of the sheep and on the strain of scrapie present. Genotypes of sheep resistant to one strain of scrapie may be susceptible to another strain but on the whole the ARR allele confers resistance in all breeds. In 2001 the UK government set up the National Scrapie Plan NSP) which aims to increase the frequency of the ARR allele within UK sheep population. Since 1988 it has been illegal for ruminant derived meat and bone meal to be fed to ruminants.
References
- ↑ . Dandoy-Dron F, Guillo F, Benboudjema L, Deslys JP, Lasmézas C, Dormont D, Tovey MG, Dron M, 1998. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. Journal of Biological Chemistry, 273(13):7691-7697,48 ref.
- ↑ Brown P, Cathala F, Raubertas RF, Gajdusek DC, Castaigne P, 1987. The epidemiology of Creutzfeldt-Jakob disease: conclusion of a 15-year investigation in France and review of the world literature. Neurology, 37(6):895-904.
- ↑ Harries JR, Knight R, Will RG, Cousens SN, Smith PG, Mathews WB, 1988. Creutzfeldt-Jakob disease in England and Wales, 1980-1984: a case-control study of potential risk factors. Journal of Neurology Neurosurgery and Psychiatry, 51(9):1113-1119.
- ↑ Kondo K, Kuriowa Y, 1982. A case control study of Creutzfeldt-Jakob disease: association with physical injuries. Annals of Neurology, 11(4):377-381.
- ↑ World Health Organization, 1999. WHO consultation on public health and animal transmissible spongiform encephalopathies: epidemiology, risk and research requirements, with the participation of the Office International des Epizooties. http://www.who.int/csr/resources/publications/bse/WHO_CDS_CSR_APH_2000,Accessed 7 March 2005. http://www.who.int/csr/resources/publications/bse/en/whocdscsraph20002.pdf.
- ↑ Beekes M, McBride PA, 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neuroscience Letters, 278(3):181-184.
- ↑ Beekes M, McBride PA, Baldauf E, 1998. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. Journal of General Virology, 79(3):601-607; 20 ref.
- ↑ Heggebø R, Press CM, Gunnes G, Lie KaiInge, Tranulis MA, Ulvund M, Groschup MH, Landsverk T, 2000. Distribution of prion protein in the ileal Peyer's patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. Journal of General Virology, 81(9):2327-2337; 2 pp. of ref.
- ↑ Kimberlin RH, Walker CA, 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Research, 12(3):213-220; 32 ref.
- ↑ Keulen LJMvan, Schreuder BEC, Vromans MEW, Langeveld JPM, Smits MA, 1999. Scrapie-associated prion protein in the gastro-intestinal tract of sheep with natural scrapie. Journal of Comparative Pathology, 121(1):55-63; 24 ref.
- ↑ Dickinson AG, Stamp JT, Renwick CC, 1974. Maternal and lateral transmission of scrapie in sheep. Journal of Comparative Pathology, 84(1):19-25.
- ↑ Hourrigan JL, Klingsporn AI, Clark WW, DeCamp M, 1979. Epidemiology of scrapie in the US. In: Prusiner SB, Hadlow W, eds. Slow transmissible diseases of the nervous system. New York: Academic Press, 331-356.
- ↑ Pattison IH, Hoare MN, Jebbett JN, 1972. Spread of scrapie to sheep and goats by oral dosing with foetal membranes from scrapie-affected sheep. Veterinary Record, 90(17):465-468.
- ↑ Fraser H, 1976. The pathology of a natural and experimental scrapie. Frontiers of Biology, 44:267-305.
- ↑ Zlotnik I, Rennie JC, 1958. A comparative study of the incidence of vacuolated neurones in the medulla from apparently healthy sheep of various breeds. Journal of Comparative Pathology, 68:411-415.
- ↑ OIE, 2000. Scrapie. OIE Manual of Standards for diagnostic tests and vaccines. 4 ed. Paris, France: Office International des Epizooties, 873-880.
- ↑ Canadian Food Inspection Agency, 2005. Scrapie. http://www.inspection.gc.ca/english/anima/heasan/man/scrtre/scrtree.shtml, Accessed 7 March 2005.
- ↑ Dawson M, Hoinville LJ, Hosie BD, Hunter N, 1998. Guidance on the use of PrP genotyping as an aid to the control of clinical scrapie. Veterinary Record, 142(23):623-625.
- ↑ European Commission, 2001. Commission Regulation (EC) No. 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Official Journal of the European Communities, L 147:1-40.
- ↑ USDA, 2005. Scrapie program. http://www.aphis.usda.gov/vs/nahps/scrapie/, accessed 7 March 2005.
| This article is still under construction. |