Major Histocompatability Complexes
|
|
Introduction
T-cells rely on Major Histocompatability Complexes (MHC) to present antigen fragments for their recognition. MHC has evolved to form two classes for antigen presentation: MHC I presents digestion fragments from antigen in cellular cytoplasm, and MHC II presents digestion fragments from antigen in the tissue fluid. As such, MHC I tends to bind slightly smaller peptides (~9 amino acids) than MHC II (~15 amino acids).
Classes
MHC I
Structure
- MHC class I is expressed on virtually all nucleated cells
- MHC class I consists of a membrane-associated heavy chain bound non-covalently with a secreted light chain
- Heavy chain:
- Made up of three distinct extracellular protein domains
- α1, α2 and α3
- The C- terminus is cytoplasmic
- Made up of three distinct extracellular protein domains
- Light chain:
- Known as β2-microglobulin
- Similar in structure to one of the heavy chain domains
- Not membrane associated
- But binds to the α3-domain of the heavy chain
- Heavy chain:
- The MHC class I domains are structurally and genetically related to immunoglobulin and TcR domains
- The outer domains (α1 and α2) are like the variable domains
- The α3 domain and β2m are like thrconstant domains
- MHC class I molecules are folded to form specific 3-dimensional structures
- The α1 and α2 domains are folded to produce an antigen-binding groove
- This groove can bind molecules of a limited size only (8-10 amino acids)
- This limits the size of epitope seen by the T-cell receptors
- This groove can bind molecules of a limited size only (8-10 amino acids)
- The α1 and α2 domains are folded to produce an antigen-binding groove
Presentation Pathway
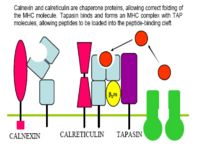
- Viral proteins are broken down to peptides by the proteasome and transferred to the endoplasmic reticulum via TAP (Transporters associated with Antigen Processing) molecules
- In the ER peptides are processed with empty MHC I molecules and exported to the cell surface for presentation
- MHC class I molecules present these to the T-cell receptors of CD8+ T-cells
MHC II
Structure
- MHC class II is expressed mainly on macrophages, dendritic cells and B-lymphocytes
- MHC class II consists of membrane-associated α and β chains
- Each chain is a transmembrane glycoprotein
- The extracellular parts of each chain have two Ig-like domains
- α1 and 7alpha;2, β1 and β2
- The outer domains (α1 and β1) are variable-like
- The inner domains (α2 and β2) are constant-like
- α1 and 7alpha;2, β1 and β2
- The 3-dimensional structure of MHC class II is similar to MHC class I
- The outer domains of the α and β chains fold in a similar way to the α1 and α2 domains of class I
- Produce the antigen-binding groove
- The outer domains of the α and β chains fold in a similar way to the α1 and α2 domains of class I
Function
- MHC class II molecules bind antigenic peptides and present them to TCR on CD4+ T-cells
- The antigen-binding groove is larger and more open than that of MHC class I
- MHC II can therefore interact with larger peptides
- MHC class II are present on those cells that have antigen-processing ability
- Interact with antigenic peptides originating from an extracellular source
- After synthesis, MHC class II molecules are transported into special endosomes
- These endosomes fuse with lysosomes that contain the digested remnants of phagocytosed microorganisms
- The peptides from the lysosome interact with the MHC class II molecules
- The peptide-MHC class II complex gets transported to the cell surface
- The peptides from the lysosome interact with the MHC class II molecules
- These endosomes fuse with lysosomes that contain the digested remnants of phagocytosed microorganisms
Interaction of MHC With Antigen
- The MHC molecules do not recognise specific amino acid sequences of antigens
- Instead, they recognise particular motifs of amino acids
- The association of any MHC allele with a peptide may be determined by the presence of as few as two amino acids
- However, these determinants must be present within a particular array
- The actual identity of the amino acids in not important for MHC binding
- Instead, the physical and chemical characteristics of the amino acid are vital
- Interactions of individual amino acids at the head and tail of the peptide-binding groove control the binding of peptides
- Are mainly positioned at the floor of the antigen-binding groove, or within the helices facing into the groove
- These MHC amino acids associate with amino acids near the ends of the peptides
- The intervening stretch of peptide folds into a helix within the groove
- Is the target for T cell receptor recognition
TCR-MHC Interaction
- Only peptide associated with self-MHC will interact with and activate T-cells
- T-cells cannot be activated by a peptide on a foreign cell
- T-cells will react against foreign MHC molecules
- This is the basis of graft rejection
The Genetics of the MHC
- Different individuals have different critical amino acids within the MHC
- I.e. different amino acids that determine peptide binding
- This variation is termed MHC polymorphism
- There are millions of variations in antibodies and TCR
- However, with MHC there is very limited variation between molecules
- MHC polymorphism has been best studied in the human
In the Human
- Humans express:
- Three types (loci) of MHC class I molecules
- HLA (Human Leukocyte Antigen)- A, B, and C
- Three loci of MHC class II molecules
- HLA-DP, DQ and DR
- Three types (loci) of MHC class I molecules
- In the entire human population there are only approximately 50 different variants (alleles) at each MHC class I and class II locus
- The variation within MHC class I is entirely on the class I heavy chain
- The β2m is invariant
- The variation within MHC class II is mainly within the β chains
- The variation within MHC class I is entirely on the class I heavy chain
- Every individual has two alleles at each MHC locus
- One inherited from each parent
- Any individual will therfore express two variants at most at each locus
- This gives a maximum variability for an individual of:
- 6 different variants of MHC class I
- 2 each of HLA- A, B and C
- 6 different variants of MHC class II
- 2 each of HLA- DP, DQ and DR
- 6 different variants of MHC class I
- This gives a maximum variability for an individual of:
- Many animal species have fewer loci than the human
- E.g. ruminants have no MHC class II DP
MHC and Disease
- Antigen from a pathogen has to be seen by the host MHC before an efficient immune response can occur
- There is therefore a constant evolutionary battle between the host and the pathogen
- There is selective pressure on the pathogen to evolve proteins that do not interact with the host MHC
- There is selective pressure on the host to continue to recognize the pathogen
- There is therefore a constant evolutionary battle between the host and the pathogen
- The consequence of this parallel evolution is that host-pathogen relationships can lead to the selection of particular MHC variants, for example:
- MHC class II alleles DR13/DR1*1301 are prevalent in Central and Western Africa
- Impart resistance to malaria
- MHC-DRB1 is prevalent in Western Europe, but rare in the Inuit populations of North America
- Associated with the clearance of hepatitis B infection in Western Europe
- Inuits have the highest incidence of hepatitis B in the world
- In humans there are also strong associations between certain alleles and some autoimmune diseases, for example:
- Diabetes mellitus
- Ankylosing spondylitis
- Rheumatoid arthritis
- MHC class II alleles DR13/DR1*1301 are prevalent in Central and Western Africa