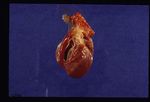
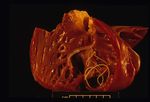
Dirofilaria immitis
| This article is still under construction. |
Also known as: heartworm.
Do not confuse with: Angiostrongylus vasorum, angiostrongylosis.
Description
Dirofilaria immitis is a nematode parasite that causes heartworm disease in dogs, cats and ferrets. Heartworm disease is transmitted by mosquito bites and there are more than 70 species of mosquito that are able to transmit infection; Aedes, Anopheles and Culex are the most common vector species. Heartwoem disease has been reported in many countries with temperate and is particularly prevalent in the USA, Canada, and southern Europe. The introduction of the PETS travel scheme has increased the concern over Dirofilariasis in the UK.
Dirofilarias does have zoonotic potential: infected mosquitos can transmit Dirofilaria immitis to humans, but the infection does not become patent. The infective larvae instead reach the lungs, become encapsulated, and die causing granulomatous reactions called "coin lesions" in the process. These are only imporant because they may be confused with neoplastic metastasis to the lungs on radiography1.
Life Cycle
Dirofilaria immitis adults reach maturity and sexually reproduce in the pulmonary arteries and right ventricle. Adult males are around 15cm in length, and females are around 25cm1. After mating, female worms release larvae known as microfilariae (or L1) into the circulation. When a mosquito takes a blood meal from the infected dog or cat, microfilariae are ingested. Mosquitos are true intermediate hosts for Dirofilaria immitis, since microfilariae require a period of maturation to L2 then L3 in the vector. The duration of this development depends upon environmental conditions. For example, maturation at 30°C takes around 8 days, but when temperatures are down to 18°C, this takes around one month2. Below 14°C, development is halted and resumes when temperatures rise. In cooler climates, this means that transmission of heartworm disease to new canine or feline hosts can only occur in warmer months.
Once matured, L3 in the mosquito migrate to the labium, from which they erupt onto the host's skin as the mosquito feeds. Larvae then migrate into the bite wound and, as most dogs are highly susceptible to heartworm disease, most L3 then establish infection. It takes 2-3 days for L3 to moult to L4, which remain in the subcutaneous tissues for up to two months before becoming young adults (L5) and migrating to the pulmonary arteries.
Cats differ from dogs in that they are more resistant to infection with Dirofilaria immitis. A lower percentage of exposed cats develop adult infections, and when this does occur the burden is usually low1. L5 in the pulmonary arteries also have a relatively short (2 year) survival time in cats.
Pathogenesis
Heartworm disease primarily affects the cardiopulmonary system and the severity and extent of lesions depends several factors. These include the number and location of adult worms1, 2, the duration of infection, and the level of activity of the host1. Parasites in the pulmonary arteries cause mechanical irritation, leading to endothelial damage, proliferation of the intima and perivascular cuffing with inflammatory cells. This results in narrowing and occlusion of the vessels which in turn causes pulmonary hypertension. A combination of pulmonary hypertension and inflammatory mediators can lead to in an increase in the permeability of pulmonary vessels, giving periarterial oedema and intersitial and alveolar infiltrates. Eventually, irreversible interstitial fibrosis arises.
Sequelae to heartworm infection include pulmonary thromboembolism, which can either occur due to the death and metastasis of adult worms, or due to platelet aggregation induced by the parasite. In severe cases, live nematodes can migrate to the right ventricle, right atrium and caudal vena cava. The resulting incompetence of the tricuspid valve, augmented by concurrent pulmonary hypertension, leads to signs of right-sided heart failure. Flow of erythrocytes through the mass of parasites formed can also cause haemolysis and thus haemoglobinaemia. This combination of acute right-sided heart failure and intravascular haemolysis is referred to as "caval syndrome", which in severe cases can also be characterised by thromboembolic events and disseminated intravascular coagulation. Due to the smaller numbers of adult worms, caval syndrome is less common in cats2.
In cats, heartworm disease generally causes a diffuse pulmonary infiltrate and an eosinophilic pneumonia2. Adult worms may die and embolise to the lungs, resulting in severe haemorrhage and oedema of the affected lobe. Immature nematodes have also been known to migrate to sites other than the pulmonary arteries and heart such as the CNS, eye and subcutaneous tissues. These ectopic infections are far more common in cats than in dogs, suggesting that D. immitis is not well adapted to feline hosts.
Signalment
Dirofilaria immitis infection affects dogs more commonly than cats, and risk is greatest in outdoor animals. Dogs of any age may be affected, but infections are most common in 3 to 8 year old dogs, and medium and large breeds are over-represented1, 3. In cats, there are no breed or age predispositions, but males are more frequently affected3. Ferrets may also contract dirofilariasis; there are no age or sex predilections1.
Diagnosis
Diagnosis:
- Physical examination:
- signs of heart disease
- lung involvement
- Radiography:
- enlargement of right heart, main pulmonary arteries; arteries in lung lobes with thickening and tortuosity; inflammation in surrounding tissues
- ECG:
- right axis deviation → deep S waves
- Echocardiography:
- if post caval syndrome suspected - right ventricular enlargement with worms in ventricle appearing as parallel lines.
Clinical pathology:
- needed alongside physical examination and other tests to determine treatment strategy and prognosis.
Parasite detection:
- methods for demonstrating microfilariae in blood:
- wet blood smear (okay for quick look, but insensitive) = D. immitis not progressively motile
- Knott's test = red blood cells lysed; stained sediment examined
- micropore filter = blood forced through; microfilariae held on filter; stained and examined
- antibody detection ELISA = not reliable in dogs, but it is the best for cats (although some false positives)
- antigen detection ELISA (using specific antigen from adult female worm) = reliable positives from 5-7months post-infection in dogs; although occasional false negatives occur → not useful for cats
- the immunochromatographic test (ICT) uses coloured gold colloidal particles tagged to monoclonal antibodies to visualise the presence of adult worm antigen - performance similar to antigen detection ELISA, but quicker and easier to do (but not as quantitative as some ELISAs are)
- operator error can give false positives, therefore best to confirm result with another test.
Clinical Signs
In dogs, infection should be identified by serologic testing prior to the onset of clinical signs; however, it should be kept in mind that HW antigenemia and microfilaremia do not appear until ~5 and 6.5 mo postinfection, respectively. When dogs are not administered a preventative and are not appropriately tested, clinical signs such as coughing, exercise intolerance, unthriftiness, dyspnea, cyanosis, hemoptysis, syncope, epistaxis, and ascites (right-sided CHF) are likely to develop. The frequency and severity of clinical signs correlate to lung pathology and level of patient activity. Signs are often not observed in sedentary dogs, even though the worm burden may be relatively high. Infected dogs experiencing a dramatic increase in activity, such as during hunting seasons, may develop overt clinical signs. Canine HW disease can be classified by physical examination, thoracic radiographs, urinalysis, and PCV. Class I is asymptomatic to mild HW disease, with no clinical or radiographic signs and no laboratory abnormalities. Subjective signs such as loss of condition, decreased exercise tolerance, or occasional cough might be seen. Class II is moderate HW disease, characterized by an occasional cough and mild-to-moderate exercise intolerance. A slight loss of condition, increased lung sounds, and mild to moderate radiographic changes, such as right ventricular enlargement, are present. Laboratory results may show anemia and proteinuria. Class III is severe disease variably characterized by anemia, weight loss, exercise intolerance, tachypnea at rest, severe or persistent coughing, dyspnea, hemoptysis, syncope, and ascites. Severely abnormal radiographs may show right ventricular hypertrophy, enlargement of the main pulmonary artery, and diffuse pulmonary densities. Laboratory results indicate marked anemia, thrombocytopenia, and proteinuria. Electrocardiographic evidence of right ventricular hypertrophy is often present. Class IV, also known as the caval syndrome, is characterized by sudden onset with collapse, hemoglobinuria, and respiratory distress. If surgery is not immediately instituted, this syndrome is usually fatal. nfected cats may be asymptomatic or exhibit intermittent coughing, dyspnea, vomiting, lethargy, anorexia, or weight loss. The symptoms often resemble those of feline asthma. In general, signs are most prevalent during periods when worms die, including when young adult worms arrive in the lungs. Antigen tests in cats are negative during the early eosinophilic pneumonitis syndrome, although antibody tests may be positive. Subsequently, clinical signs often resolve and may not reappear for months. Cats harboring mature worms may exhibit intermittent vomiting, lethargy, coughing, or episodic dyspnea. HW death can lead to acute respiratory distress and shock, which may be fatal and appears to be the consequence of pulmonary thrombosis.
Diagnostic Imaging
In dogs, echocardiography is relatively unimportant as a diagnostic tool. Worms observed in the right heart and vena cava are associated with high-burden infection with or without caval syndrome. Severe, chronic pulmonary hypertension causes right ventricular hypertrophy, septal flattening, underloading of the left heart, and high-velocity tricuspid and pulmonic regurgitation. The ECG of infected dogs is usually normal. Right ventricular hypertrophy patterns are seen when there is severe, chronic pulmonary hypertension and are associated with overt or impending right-sided CHF (ascites). Heart rhythm disturbances are usually absent or mild, but atrial fibrillation is an occasional complication in dogs with Class III disease.
In cats, worms can usually be imaged on echocardiography. Parallel hyperechoic lines, which are an image from the heartworm cuticle, may be seen in the right heart and pulmonary arteries. High worm burdens may be associated with worms in the right heart. Echocardiography is more important in cats than dogs because of the increased difficulty of diagnosis and the high sensitivity of the test in experienced hands.
n dogs, thoracic radiography provides the most information on disease severity and is a good screening tool for dogs with clinical signs compatible with dirofilariasis. Class III infections are characterized by a large main pulmonary artery segment and dilated, tortuous caudal lobar pulmonary arteries. If the latter are ≥1.5 times the diameter of the 9th rib at their point of superimposition, then severe pathology is present. Right ventricular enlargement may also be seen. Fluffy, ill-defined parenchymal infiltrates of variable extent often surround the caudal lobar arteries, usually worst in the right caudal lobe, in advanced disease. The infiltrate may improve with cage confinement with or without anti-inflammatory dosages of a corticosteroid. In cats, cardiac changes are less common. The caudal lobar arteries normally appear relatively large, but are larger still with heartworm infection. Patchy parenchymal infiltrates may also be present in cats with respiratory signs. The main pulmonary artery segment usually is not visible due to its relatively midline location.
Laboratory Tests
In addition to special diagnostic tests in both cats and dogs, a CBC, chemistry profile, urinalysis, and particularly thoracic radiographs are indicated. Laboratory data are often normal. Eosinophilia and basophilia are common and together suggest occult dirofilariasis or allergic lung disease. Eosinophilia surges as the L5 arrive in the pulmonary arteries. Subsequently, eosinophil counts vary but are usually high in dogs with immune-mediated occult infections, especially if eosinophilic pneumonitis develops (<10% of total infections). Hyperglobulinemia may be present in dogs and cats due to antigenic stimulation. Hypoalbuminemia in dogs is associated with severe immune-complex glomerulonephritis or right-sided CHF. Serum ALT and alkaline phosphatase are occasionally increased, but do not correlate well with abnormal liver function, efficacy of adulticide treatment, or risk of drug toxicity. Urinalysis may reveal proteinuria that can be semiquantitated by a urine protein:creatinine ratio. Occasionally, severe glomerulonephritis or amyloidosis can lead to hypoalbuminemia and nephrotic syndrome. Dogs with hypoalbuminemia secondary to glomerular disease also lose antithrombin III and are at risk for thromboembolic disease. Hemoglobinuria is associated with Class III disease when RBC are lysed in the pulmonary circulation by fibrin deposition. Heparin therapy (75-100 U/kg, SC, tid) is indicated. Hemoglobinuria is also a classic sign of the vena caval syndrome.
he antigen detection test is the preferred diagnostic method for asymptomatic dogs or when seeking verification of a suspected HW infection. This is the most sensitive diagnostic method available to veterinary practitioners. Even in areas where the prevalence of HW infection is high, ~20% of infected dogs may not be microfilaremic. Also, monthly macrolide prophylaxis induces embryo stasis in female dirofilariae. Available antigen detection tests are very sensitive and specific. To determine when testing might become useful, it is advisable to add a predetection period to the approximate date on which infection may have been possible. A reasonable interval is 7 mo. There is generally no need to test a dog for antigen or microfilariae prior to ~7 mo of age. The level of antigenemia is directly related to the number of mature female worms present. At least 90% of dogs harboring ≥3 adult females will test positive. In general, strong-quick positive reactions correlate with relatively high worm burdens. For low-burden suspects, commercial laboratory-based microwell titer tests are the most sensitive.
Pathology
On post-mortem examination, the right side of the heart is found to be enlarged. There is proliferation of the pulmonary arterial myointima, as well as pulmonary thromboembolism and haemorrhage. If right-sided congestive heart failure was present in life, the hepatomegaly and hepatic congestion will be seen.
Treatment
ADULTICIDAL TREATMENT The decision as to whether or not to treat a dog with heartworm disease and the relative prognosis depend on the severity of the infection. In an attempt to assess this, animals may be classified as:
- CLASS 1. Patients with subclinical or no clinical signs;
- CLASS 2. Patients with mild to moderate clinical signs
and mild to moderate radiographic changes;
- CLASS 3. Patients with severe clinical signs (eg, persistent
cough, haemoptysis, dyspnoea, right-sided heart failure) and severe radiographic changes. The prognosis for dogs in class 1 following adulticidal treatment is generally very good. Dogs in class 2 may have a positive outcome. Animals in class 3 have a guarded prognosis due to the high risk of pulmonary thromboembolism; in these cases, the potential complications of adulticidal treatment should be thoroughly discussed with the owners. In cats, adulticidal treatment may be dangerous even in patients with low grade infection, and pulmonary thromboembolism due to premature death of parasites is a common complication. Considering that some cats may undergo spontaneous clinical remission if parasites die naturally, periodic monitoring of the patient is more appropriate than adulticidal treatment. Cats with respiratory signs can be treated symptomatically in the same way as patients with feline asthma. Cage rest, oxygen supplementation, fluid therapy, bronchodilators and injectable steroids (eg, dexamethasone) can be used to stabilise acutely ill cats. Adulticidal treatment should only be considered for cats in a stable condition that fail to respond to supportive treatments. Adulticidal treatment involves the administration of a new generation arsenical compound, melarsomine dihydrochloride (Immiticide, Merial; this product is not licensed for use in the UK). Melarsomine is less nephrotoxic and hepatotoxic than its predecessor thiacetarsamide, and has a higher efficacy. Melarsomine is injected intramuscularly into the lumbar muscles at a recommended dose of 2-5 mg/kg, repeated after 24 hours. However, only a single dose should be administered to class 3 dogs, to kill just a proportion of worms and hence minimise the risk of pulmonary thromboembolism. If the patient remains stable, the standard adulticidal protocol can be administered one month later. In the week following the administration of melarsomine, the likelihood of pulmonary thromboembolism can be minimised with cage rest and corticosteroids at anti-inflammatory doses. If adulticidal treatment is declined by the owner, monthly administration of prophylactic doses of ivermectin may represent a reasonable option because it will prevent further infection and may kill some adult nematodes. Patients with severe caval syndrome may benefit from physical removal of worms from the right side of the heart and the main pulmonary artery using flexible crocodile or basket-type retrieval forceps. This procedure is complex, requires general anaesthesia and fluoroscopic imaging, but may reduce the risk of thromboembolism following subsequent adulticidal treatment. MICROFILARICIDAL TREATMENT Circulating microfilariae should be eliminated four to six weeks after successful adulticidal treatment. There are no approved drugs for microfilaricidal treatment. However, a single administration of ivermectin (50 jig/kg) or milbemycin oxime (500 jig/kg) has been shown to be highly effective in eliminating microfilariae from the circulation within a few hours. Moxidectin and selamectin are also known to be potent microfilaricides but, at present, there is little experience of their use in the clinical setting. Oral prednisolone (1 to 2 mg/kg) administered with microfilaricidal drugs may control anaphylactic reactions which may occur secondarily to the sudden death of a large number of microfilariae. PREVENTION Prophylaxis of heartworm disease should be considered for all dogs and cats living in endemic areas during the transmission period. For example, in northern Italy, where a high prevalence of heartworm disease has been reported, mosquitoes are only present during the summer and, therefore, prophylaxis is recommended from May to October. The currently approved prophylactic drugs are listed in the table below. However, only milbemycin oxime (Program Plus; Novartis) and selamectin (Stronghold;Pfizer) are licensed in the UK for the prevention of heartworm disease. Preventive drugs kill migrating larvae of D immitis up to the sixth week of infection. Therefore, they all provide a high degree of protection when administered on a monthly basis. If a patient misses one or more doses in the prophylactic schedule, it should be tested for heartworm disease after six months. The risk of incomplete protection due to owner noncompliance can be eliminated with a recently introduced sustained-release injectable formulation of moxidectin (Proheart 6; Fort Dodge). High doses of ivermectin and milbemycin oxime are potentially toxic in approximately one-third of collies. However, side effects are not observed in these breeds when prophylactic drugs are administered at the recommended doses.
Prognosis
Links
References
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Ferasin, L (2004) Disease risks for the travelling pet: Heartworm disease, In Practice, 26(6), 350-357.
- Tilley, L P and Smith, F W K (2004) The 5-minute Veterinary Consult (Fourth Edition),Blackwell.
- Ridyard, A (2005) Heartworm and lungworm in dogs and cats in the UK, In Practice, 27(3), 147-153.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in cats. Dirofilaria immitis and D. repens in dog and cat and human infections, 126-132.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in dogs. Dirofilaria immitis and D. repens in dog and cat and human infections, 117-125.