Bovine Viral Diarrhoea Virus
| This article is still under construction. |
Description
Bovine viral diarrhoea is a viral disease that affects cattle worldwide. Caused by a pestivirus, it gives rise to significant economic losses in both dairy and beef cattle through its effects on production and reproduction. Bovine viral diarrhoea virus can lead to a variety of clinical outcomes that ranging from subclinical infections to the more severe presentations including abortion, infertility, and the fatal mucosal disease. The condition is highly immuno-suppressive and secondary respiratory and enteric complications often occur.
Bovine Viral Diarrhoea Virus
Classification
The viral aetiology of BVD was first established over 60 years ago, but it was not until the 1960s that the agent was assigned to the newly penned "Pestivirus" genus. At this stage Pestiviruses were considered to be non-arthropod-borne togaviruses; later, sequencing of genomic RNA showed that they are taxonomically better suited to the Flaviviridae family1, 2. Many members of the Flaviviridae family are indeed arthropod-borne, such as the Flaviviruses West Nile Virus and yellow fever virus. However, Pestiviruses are not transmitted by insects, and the genus includes pathogens of cattle (BVDV), sheep (Border Disease virus) and pigs (Classical Swine Fever Virus).
Virus Structure
The BVDV genome comprises a single strand of positive sense RNA which is around 12.3 kilobases in length3. The genome is read in one 3898-codon open reading frame that contains no non-coding sequences. BVDV polyprotein is translated directly from the ORF and is cleaved by viral and cellular proteinases to form mature viral proteins3, 4. At either end of the ORF, 5’ and 3’ untranslated regions exist. These regions are long, allowing them to accomodate fuctions conferred in eukaryotic DNA by the 5’ cap and the 3' poly-A tail, such as controlling the initiation of translation, facilitating the entry of replicases, and contributing to RNA stability4. BVDV's RNA genome encodes both structural and non-structural proteins. These include Npro, whose protease action generates the N-terminus of the protein C. C is the capsid protein that packages genomic RNA and assists in the formation of the eventual enveloped virion. Erns, E1 and E2 are all glycoproteins, with Erns possessing RNase activity involved in viral replication and pathogenesis5. E1 is membrane-anchored and initiates the translocation of the antigenic protein E2 to the envelope3. P7 has an uncertain function6. NS2-3 is the first non-structural protein to be translated. Sequence similarities are shown by NS2-3 to a region that in other Flaviviridae is split into two distinct polypeptides, NS2 and NS3. In BVDV, NS2 and NS3 can be expressed as separate polypeptides: NS3 is found exclusively in cytopathic isolates from 6 hours post-infection, making it a marker of this biotype3. NS2 is also expressed as a discrete polypeptide in some cytopathic isolates. NS2-3, along with the other non-structural proteins, plays an important role in genome replication. A serine protease domain within NS2-3 functions to release NS4A, NS4B, NS5A and NS5B7. NS4A is a cofactor for the serine protease7, and NS5B possesses an RNA-dependent RNA polymerase activity8. Knowledge of the role of NS4B is limited.
Newly formed genomic material is packaged by structural proteins to create the BVDV virion which is 40-60nm in diameter. The capsid is surrounged by a membrous envelope, in which the glycoproteins E1 and E2 are anchored. Naked BVDV RNA is infectious3, 4, and so it can be deduced that the virions do not contain enzymes necessary for RNA replication: these are provided by the host cell.
Virus Genotypes
There are two antigenically distinct9 genotypes of BVDV, type 1 and type 2, which are most accurately characterised based on sequence variation. BVDV-1 and BVDV-2 contain 11 and 3 subtypes respectively, which have been demonstrated by analysis of the 5’UTR10. Despite the large antigenic differences between the genotypes, cross-protection against type 2 viruses is afforded by type 1 vaccines11, 12, 13.
In general, the genotypes differ in virulence. BVDV-1 species are found worldwide and tend to cause milder disease 14, whereas BVDV-2 isolates typically cause more severe disease which is often haemorrhagic and is associated with a high mortality rate11, 15. Type 2 viruses were first reported in Canada and the USA and have a more limited distribution than type 1 isolates. The relationship between genotype and virulence is, however, not fixed: some type 2 strains cause mild or subclinical disease16, and the spectrum disease caused by type 1 viruses is broad.
Virus Biotypes
BVDV isolates from either genotype can be of a cytopathic or non-cytopathic biotype. Non-cytopathic (ncp) viruses produce no visible effects in cell culture, whereas infection with cytopathic (cp) viruses gives cell vacuolation and death. Although non-cytopathic isolates are responsible for the majority of BVDV infections worldwide, cytopathogenicity gives no indication of disease-causing potential. Cytopathic biotypes of bovine viral diarrhoea virus are always isolated alongside non-cytopathic strains, and are found in cases of mucosal disease, a fatal BVD-associated condition.
Cytopathic viruses originate from non-cytopathic strains by mutation, including viral gene rearrangements, duplications and deletions17, and insertions of cellular origin, such as ubiquitin sequences. Point mutations in the NS2 region and various RNA recombination events are also important18. Serologically, the two BVDV biotypes are indistinguishable, but on a molecular level cytopathic viruses produce an additional protein, NS3, not found in cells infected with non-cytopathic virus19, 20, 21. This marker molecule arises from when NS2-3, expressed in non-cytopathic isolates, is cleaved at a site created by the mutations above22.
Transmission and Epidemiology
In most countries, BVDV is endemic and studies detecting antibody have estimated that between 70 and 100% of herds are either currently infected or have recently been infected with bovine viral diarrhoea virus23.
BVDV can be transmitted from infected to susceptible cattle in several ways. Firstly, direct contact with an animal shedding BVDV in its secretions and excretions can cause disease. Virus is shed by both acutely and persistently infected (PI) animals but levels of shedding are much higher in persistently infected cattle, which are a natural reservoir for virus. It is estimated that the incidence of persistently infected animals may be as high as 1-2% of cattle less than one year of age. On a farm, PI cattle are often found in cohorts of similarly aged animals. This is because persistent infections arise when pregnant animals are acutely infected, and so an outbreak of acute, possibly subclinical, BVD in pregnant cattle can later result in a "batch" of PI calves.
Transmission to heifers and cows may also occur venereally or via artificial insemination as acutely and persistently unfected bulls sheed bovine viral diarrhoea virus in their semen24. The testes is an immunoprivileged site, and the virus can persist in this location despite otherwise systemic clearance25. Indirect spread is possible: BVDV has been shown to spread through the re-use of needles, nose tongs26 and rectal gloves27, and blood feeding flies also give transmission.
Pathogenesis
Following entry and contact with the mucosa or the oral or nasal cavity, or the reproductive tract, BVDV replicates in epithelial cells and has a predilection for the palatine tonsil and the nasal mucosa. From here, the virus spreads to regional lymph nodes before a viraemia becomes established. Virus can be disseminated free in the blood, or associated with leukocytes, particularly lymphocytes and monocytes28. Bovine viral diarrhoea virus can then gain access to many tissues, but shows a preference for lymphoid tissue, reaching its highest concentrations in the tonsil, thymus and ileum. Bone marrow29 and intestinal mucosa are often infected, and the lymphoid tissue of the Peyer's patches frequently depleted. however, there is variation between strains as to which tissues are specifically infected and in generally, a wider distribution is associated with higher virulence.
Diagnosis
Although the clinical appearance may suggestive of BVD, disease presentation can vary widely and so laboratory testing is usually necessary.
Clinical Signs
The disease caused by bovine viral diarrhoea virus is known as bovine viral diarrhoea. It might be expected from this nomenclature that diarrhoea is a key clinical feature in BVDV infection, but disease can actually manifest in a variety of ways, ranging from subclinical disease to muscosal disease. Virulence factors related to genotype and strain are partially responsible for these variations, but host factors are also important. Pregnancy status, stage of gestation, immunity and the level of develoment of the foetal immune system all contribute to the outcome of BVDV infection.
Acute Infections: Non-Pregnant Cattle
In the naive, non-pregnant, immunocompetent animal, BVD is normally mild: it is estimated that 70 to 90% of BVDV infections cause no clinical signs30. If these subclinically affected cattle are observed closely, body temperature may marginally rise and mild leukopenia and agalactia may be seen 31, 32. When clinical disease does occur in these animals, morbidity is high amongst cattle of 6-12 months of age. Following a 5-7 day incubation period, pyrexia and leukopenia is seen. Viraemia arises on days 4-5 days post-infection, and continues until around day 1533. Although some cattle suffer diarrhoea in BVDV infection, the disease no longer seems to present as herd outbreaks of diarrhoea34. Clinical signs more commonly include depression, anorexia, occulo-nasal discharge, decreased milk production and oral lesions35, with a rapid respiratory rate resembling pneumonia sometimes apparent31. Acutely infected, non-pregnant animals shed low concentrations of virus compared to persistently infected cattle33, and antibodies are produced 2-4 weeks post-infection which persist for life35.
Acute BVDV infection causes a significant leukopenia, hampering the host's defences against invading pathogens. This BVDV-associated immunosupression has a particularly important role in bovine respiratory disease: an association has been demonstrated between BVDV antibody titre and treatment for respiratory disease36. BVDV is the virus most frequently isolated from pneumonic lungs and is often found in association with Pasteurella haemolytica35, causing severe fibrino-purulent bronchopneumonia with the total lesion area increased by 35-60% to that caused by pasteurellosis alson34 Synergism is also displayed with parainfluenza, bovine rhino-tracheitis and respiratory syncitial viruses.
Although BVDV infections in naive, non-pregnant animals are usually mild, outbreaks of a severe form of BVD have been known11, 37. These were characterised by the acute onset of diarrhoea, pyrexia and milk drop, with some cases proving fatal. These oubtreaks were associated with genotype 2 viruses, and it transpired vaccintion with type 1 vaccines had not afforded cross-protection in these instances due to non-compliance with instructions. Generally, BVDV-2 infection is seen less frequently than disease related to type 1 virus, but is associated with haemorrhagic syndrome. Haemorrhagic syndrome is characterised by severe thrombocytopaenia leading to haematochezia, petechiation and epistaxis38 and has been described in both Europe and North America. Severe disease is also possible with virulent type 1 infection, presenting as high fever, oral ulcerations, eruptive lesions of the coronary band and interdigital cleft, diarrhoea, dehydration, leukopenia, and thrombocytopenia. Thrombocytopenia may give petechiation of the conjunctiva, sclera, nictitating membrane and the mucosal surfaces of the mouth and vulva, as well as prolonged bleeding from injection sites39.
Acute Infections: Pregnant Animals
When acute BVDV infection occurs during pregnancy, the dam may show any of the clinical manifestations that are seen in non-pregnant animals. BVDV is able to cross the placenta and infect the developing foetus and so there may be additional outcomes of infection that depend on the stage of gestation. If infection becomes established at the time of insemination, conception rates may be reduced, and early embryonic death is increased when the virus is introduced at a slightly later stage40, 41. Foetal infection in the first trimester (50-100 days) may also result in death, although expulsion of the foetus often does not not occur until several months later.
Congenital defects can arise from transplacental infection between days 100 and 150. This is caused by an inappropriate inflammatory response mounted to BVDV by the immune system, which is undergoing the final phase of development at this stage33. Examples of common congenital abnormailites include defects of the thymus, occular changes and cerebellar hypoplasia34. Calves with cerebellar hypoplasia ataxic, reluctant to stand and may suffer tremors35, and occular pathology often causes blindness and cataracts. Localisation of virus to the vascular endothelium gives vasculitis, leading to oedema, hypoxia and cellular degeneration. Weak, stunted calves may also be produced by BVDV infection in the second trimester.
Infection in the third trimester trimester (over 180-200 days) elicits a response from the fully-developed immune system, giving rise to normal but seropositive calves. An additional effect of foetal infection before 120 days gestation is the birth of persistently infected calves.
Persistent Infections
Foetal infection with a non-cytopathic BVDV virus before 120 days gestation may result in the birth of calves pesistently infected with and tolerant to bovine viral diarrhoea virus. At this stage in gestation, the immune system is partially competent and recognises the BVDV antigen as self, meaning that response is mounted. The calf therefore becomes tolerant to the virus, which persists into neonatal life. Persistently infected animals can be identified at birth as being antigen-positive but seronegative. However, colostral transfer of maternal immunity through colostrum or infection with a heterologous strain of BVDV can will make these animals seropostitive, so care must be taken when timing and interpreting tests42.
Persistently infected animals continuously shed large amounts of virus throughout their lives, providing a major source of infectious virus for naïve cattle23. Persistently infected dams produce persistently infected calves, resulting in family lines capable of maintaining the virus in a herd35. It is estimated that 1-2% of the cattle population up to 13% of foetal calves are persistently infected34.
50% of persistently infected cattle die within the first year of life33. Animals may be undersized and slow-growing, and are predisposed to other diseases43. Persistent infection with BVDV is the prerequisite for developing mucosal disease.
Mucosal Disease
Mucosal disease is an invariably fatal condition of 6-18 month-old cattle44. Disease follows a course of several days to weeks, and intially presents as pyrexia, depression and weakness. Anorexia leads to emaciation, and animals suffer profuse, watery, foul-smelling and sometimes bloody diarrhoea. Dehydration ensues. As suggested by the name, lesions are localised to mucosal surfaces. These include the oral mucosa, tongue, external nares, nasal cavities and conjunctiva34, where large lesions cause excessive salivation, lacrimation, and oculo-nasal discharge. The coronet and interdigital surface are also affected, causing the animal to become disinclined to walk and eventually recumbent.
Mucosal disease arises from superinfection of persistently infected animals with a cytopathic virus antigenically similar to the original, non-cytopathic strain persisting in the animal. In one animal, a cytopathic virus is produced by mutation of the persistent non-cytopathic virus. The new cytopathic isolate can then be transmitted to other animals, where it will cause mucosal disease if they are persistently infected with the same non-cytopathic strain. Immune tolerance induced by the pesistent virus prevents the immune system recognising the superinfecting cytopathic strain: the two biotypes are said to be "homologous" to the immunotolerace.45. "Heterologous" superinfection with a non-related cytopathic biotype does not result in mucosal disease because a normal immune response is mounted.
Laboratory Tests
There are several techniques available for the laboratory diagnosis of BVD. These can detect antibody to BVDV, or parts of the virus itself.
Tests that detect anti-BVDV antibody include the serum neutralisation test, and an ELISA34. The serum neutralisation test depends on the ability of antibodies in the serum to neutralise BVD virus and thereby prevent infection of cell culture. The test takes four to seven days to obtain a result and requires cell culture facilities and an experienced observer. The ELISA detects anti-BVDV antibody when it binds to a specifica viral antigen. The test is completed within one day and is simple to perform. Because antibody against BVDV is prevalent in most cattle populations, a single serologic test is not usually sufficient for diagnosis. Therefore, an increase in antibody titre between paired serum samples must be more than four-fold to confirm recent infection39.
Viral antigen or RNA can be detected using clinical specimens or tissue samples. Bovine viral diarrhoea virus can be isolated from blood, nasal swabs or tissues to confirm active infection, and demonstration of virus in samples obtained at least three weeks apart is suggestive of persistent infection. The best tissues for virus isolation are spleen, lymph node and segments of the gastrointestinal tract showing ulcerative lesions. An antigen-cpature ELISA is also available to detect the presence of BVDV antigen in blood or serum, and immunohistochemistry will demonstrate the presence of antigen in fixed or frozen sections. Viral RNA may also be detected, using PCR for clinical specimens and PCR of in situ hybridisation on fresh or firxed tissues39.
Genotype is generally determined by PCD or nucleic acid sequencing.
Pathology
In cases of mild, acute BVD, lesions are rarely seen. When disease is more severe, the lymph nodes may appear swollen, there may be erosions and ulcerations of the gastrointestinal tract tract and serosal surfaces of the viscera may show petechial and ecchymotic hemorrhages39.
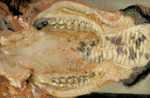
The pathology associated with mucosal disease is much more striking34. Oral, lingual and buccal erosions are observed, and buccal lesions often coalesce to form larger areas of necrosis and sloughed epithelium. Oesophageal lesions present similarly. The gastrointestinal tract often shows characteristic pathology, but post-mortem examination must be performed soon after death so that these are not masked by autolytic changes. In the rumen, ulceration is rare but congestion and oedema may be seen along the pillars, and papillae can be reduced in size. Several discoid erosions of around 5mm in diameter appear in the abomasum, with hyperaemia of the surrounding mucosa and petechiation of the submucosa, particularly at the pylorus. Abomasal erosions occasionally enlarge and ulcerate.
The small intestine, if opened throughout its length to expose the antimesenteric surface, will reveal oval erosions (2 cm to 5 cm long) that overlie the lymphatic tissue in Peyer's patches. The erosions may vary from two to three to 30 to 40. Towards the terminal ileum the erosions can become extensive and may be up to 10 to 20 cm in length. The exposed submucosal surface of the erosions can vary from the chronic lesion, with food adhering (Fig 7), to the acutely congested one often with interluminal haemorrhage. In the large bowel, there may be congestion of the mucosa which gives a thickening to the mucosal folds and a striped appearance. There may also occasionally be petechial haemorrhages and small erosions along the folds. The contents are dark, watery and often foul smelling. Gross lesions seldom are seen in cases of mild disease. (Brownlie, 1985).
Treatment and Control
Once a herd’s status has been established, BVDV control measures can be implemented. Previously, PI animals have been used as natural “vaccinators” to increase herd immunity, although naïve animals must endure acute infection before this is achieved. Eradication by identification and culling of PI animals is possible, having been successfully accomplished in Scandinavia. However, this gives many seronegative, susceptible animals which is an imperfect solution for those units not completely biosecure or highly committed to the scheme. Killed and live vaccines afford a good level of protection providing they are used correctly and boosted regularly. A combination of eradication and vaccination gives the best level of control (Brownlie et. al, 2000), and the development of “marker” vaccines that allow natural- and vaccine-induced immunity to be distinguished will help this cause in future.
- No known treatment to reverse persistent infection or to cure mucosal disease
- Beta-propiolactone inactivated vaccine
- Combine with screening for antigen and removal of PI animals
Treatment of BVD is limited primarily to supportive therapy. Control is based on sound management practices that include use of biosecurity measures, elimination of persistently infected cattle, and vaccination. Replacement cattle should be tested for persistent infection before entry into the herd. Quarantine or physical separation of replacement cattle from the resident herd for 2-4 wk should be considered, and vaccination of replacement cattle for BVD should be done before commingling with the resident herd. Embryo donors and recipients also should be tested for persistent infection. If vaccination of embryo donors or recipients is warranted, it should be done at least 1 estrous cycle before embryo transfer is performed. Because BVDV is shed into semen, breeding bulls should be tested for persistent infection before use. Artificial insemination should be done only with semen obtained from bulls free of persistent infection. Screening cattle herds for persistent infection is done by virus isolation from serum or buffy coat cells, antigen-capture ELISA from serum or buffy coat, or antigen detection in skin biopsies. Several strategies, based on herd size, type of herd being screened, financial limitations of the herd owner, and testing ability of the diagnostic laboratory being used, are available to screen herds for persistent infection. When identified, persistently infected cattle should be sold for slaughter as soon as possible. Inactivated and modified live virus vaccines are available. They contain a variety of strains of BVDV representing both viral biotypes and viral genotypes 1 and 2. Antigenic diversity among BVDV may affect the efficacy of a given vaccine if the vaccine virus or viruses differ significantly from the challenge virus. Proper and safe immunization of cattle with either inactivated or modified live virus vaccines requires adherence to the manufacturer’s instructions. Because BVDV is fetotropic and may be immunosuppressive, use of modified live virus vaccines is not recommended in cattle that are pregnant or showing signs of disease. Inactivated viral vaccines may be used in pregnant cattle. Protection conferred by inactivated vaccines may be of short duration, and frequent vaccination may be necessary to prevent disease or reproductive failure. Colostral antibody confers partial to complete protection against disease in most calves for 3-6 mo after birth. Vaccination of neonatal cattle that have acquired colostral antibody may not stimulate a protective immune response, and revaccination at 5-9 mo of age may be necessary.
Links
References
- Collett, M S et al (1988) Proteins encoded by bovine viral diarrhoea virus: The genomic organisation of a pestivirus. Virology, 165(1), 200-208.
- Meyers, G et al (1989) Molecular Cloning and nucleotide sequence of the genome of hog cholera virus. Virology, 171(2), 555-567.
- Donis, R O(1995) Molecular biology of bovine viral diarrhea virus and its interactions with the host. The Veterinary Clinics of North America: Food Animal Practice 11(3), 393-424.
- Dubovi, E J (1990) Molecular biology of bovine virus diarrhoea virus. Revue Scientifique et Technique, 9(1), 105-114.
- Van Gennip, H G P et al (2005) Dimerisation of glycoprotein Erns of classical swine fever virus is not essential for viral replication and infection. Archives of Virology, 150(1), 2271-2286.
- Tautz, N et al (1999) Establishment and Characterization of Cytopathogenic and Noncytopathogenic Pestivirus Replicons. Journal of Virology, 73(11), 9422–9432.
- Harada, T et al (2000) E2-p7 Region of the Bovine Viral Diarrhea Virus Polyprotein: Processing and Functional Studies. Journal of Virology, 74(20), 9498–9506.
- Zhong, W et al (1998) Identification and Characterization of an RNA-Dependent RNA Polymerase Activity within the Nonstructural Protein 5B Region of Bovine Viral Diarrhea Virus. Journal of Virology, 72(11), 9365–9369.
- Paton, D J et al (1995) A proposed division of the pestivirus genus using monoclonal antibodies, supported by cross-neutralisation assays and genetic sequencing. Veterinary Research, 26, 82-109.
- Vilcek, S et al (2001) Bovine viral diarrhoea virus genotype 1 can be separated into at least eleven groups. Archives of Virology, 146, 99-115.
- Carman, S et al (1998) Severe acute bovine viral diarrhea in Ontario, 1993-1995. Journal of Veterinary Diagnostic Investigation, 10, 27-35.
- Cortese, V S et al (1998) Clinical and immunologic responses of vaccinated and unvaccinated calves to infection with a virulent type-II isolate of bovine viral diarrhea virus. Journal of the American Veterinary Medical Association, 213, 1312-1319.
- Van Oirschot, J T et al (1999) Vaccination of cattle against bovine viral diarrhoea. Veterinary Microbiology, 64, 169-183.
- Deregt, D et al (2004) Attenuation of a virulent type 2 bovine viral diarrhea virus. Veterinary Microbiology, 100, 151-161.
- Corapi, W et al (1989) Severe Thrombocytopenia in Young Calves Experimentally Infected with Noncytopathic Bovine Viral Diarrhea Virus. Journal of Virology, 63(9), 3934-3943.
- Ahn, B C et al (2005) Biotype, Genotype, and Clinical Presentation Associated With Bovine Viral Diarrhea Virus (BVDV) Isolates From Cattle. International Journal of Applied Research in Veterinary Medicine, 3(4), 319-325.
- Deregt, D and Loewen, K G (1995) Bovine viral diarrhea virus: Biotypes and disease. Canadian Veterinary Journal, 36, 371-378.
- Donis, R O and Dubovi, E J (1987). Differences in virus-induced polypeptides in cells infected by cytopathic and noncytopathic biotypes of bovine virus diarrhea-mucosal disease virus. Virology, 158, 168-173.
- Pocock, D H et al (1987). Variation in the intracellular polypeptide profiles from different isolates of bovine viral diarrhea virus. Archives of Virology, 94, 43-53.
- Magar, R et al (1988). Bovine viral diarrhea virus proteins: heterogeneity of cytopathogenic and noncytopathogenic strains and evidence of a 53K glycoprotein neutralization epitope. Veterinary Microbiology, 16, 303-314.
- Tautz, N et a; (1999) Establishment and Characterization of Cytopathogenic and Noncytopathogenic Pestivirus Replicons. Journal of Virology, 73(11), 9422–9432.
- Meyers, G and Thiel, H J (1996). Molecular characterization of pestiviruses. Advances in Virus Research, 47, 53-118.
- Houe, H (1999) Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Veterinary Microbiology, 64, 89-107.
- Kirkland, P D et al (1991) Replication of bovine viral diarrhoea virus in the bovine reproductive tract and excretion of virus in semen during acute and chronic infections. Veterinary Record, 128, 587–590.
- Gunn, H M (1993) Role of fomites and flies in the transmission of bovine viral diarrhoea virus. Veterinary Record, 132, 584-585.
- Lang-Ree, J R et al (1994) Transmission of bovine viral diarrhoea virus by rectal examination. Veterinary Record, 135, 412-413.
- Tarry, D W et al (1991). Transmission of bovine virus diarrhoea virus by blood feeding flies. Veterinary Record, 128(4), 82-84.
- Bruschke, C J M et al (1998) Distribution of bovine virus diarrhoea virus in tissues and white blood cells of cattle during acute infection. Veterinary Microbiology, 64, 23-32.
- Spagnuolo, M et al (1997) Bovine Viral Diarrhoea Virus Infection in Bone Marrow of Experimentally Infected Calves. Journal of Comparative Pathology, 116, 97-100.
- Ames, T R (1986) The causative agent of BVD: Its epidemiology and pathogenesis. Veterinarni Medicina, 81, 848-869.
- Perdrizet, J A et al (1987).Bovine virus diarrhea – clinical syndromes in dairy herds. Cornell Veterinarian, 77, 46-74.
- Moerman, A et al(1994) Clinical consequences of a bovine virus diarrhoea in a dairy herd: A longitudinal study. Veterinary Quarterly, 16, 115-119.
- Duffell, S J and Harkness, J W (1985) Bovine virus diarrhoea-mucosal disease infection in cattle. Veterinary Record, 117, 240-245.
- Brownlie, J (1985) Clinical aspects of the bovine virus diarrhoea/ mucosal disease complex in cattle. In Practice, 7(6), 195-202.
- Baker, J (1995) The Clinical Manifestations of Bovine Viral Diarrhea Infection. The Veterinary Clinics of North America: Food Animal Practice, 11(3), 425-445.
- Martin, S W and Bohac, J G (1986) The association between serologic titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncitial virus and the treatment for respiratory disease in Ontario feeder calves. Canadian Journal of Veterinary Research, 50, 351-358.
- Hibberd, R C and Turkington, A (1993) Fatal bovine viral diarrhoea virus infection of adult cattle. Veterinary Record, 132, 227-228.
- Rebhun, W C et al (1989) Thrombocytopenia associated with bovine viral diarrhoea infection in cattle. Journal of Veterinary Internal Medicine, 3, 42-46.
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Carlsson, U et al (1989) Bovine virus diarrhoea virus: A cause of early pregnancy failure in the cow. Journal of Veterinary Medicine, 36, 15-23.
- Mc Gowan, M R et al (1993) Increased reproductive losses in cattle infected with bovine pestivirus aroung the time of insemination. Veterinary Record, 133, 39-43.
- Bolin, S R et al (1985) Response of cattle persistently infected with noncytoparthic bovine viral diarrhea virus to vaccination for bovine viral diarrhea and subsequent challenge exposure who cytopathic bovine viral diarrhea virus. American Journal of Veterinary Research, 46, 2467-2470.
- Houe, H (1993) Survivorship of animals persistently infected with bovine virus diarrhoea virus (BVDV). Preventative Veterinary Medicine, 115, 275-283.
- Brownlie, J et al (2000) Bovine virus diarrhoea virus- strategic decisions for diagnosis and control. In Practice, 22(4), 176-187.
- Brownlie, J (1990) Pathogenesis of mucosal disease and molecular aspects of bovine virus diarrhoea virus. Veterinary Microbiology, 23, 371-382.