Equine Togaviral Encephalitis
| This article is still under construction. |
Description
NOTIFIABLE and ZOONOTIC infectious mosquito-borne diseases of equidae affecting the central nervous system (CNS). They include:
- Eastern Equine Encephalitis (EEE)
- Western Equine Encephalitis (WEE)
- Venezuelan Equine Encephalitis (VEE)
Aetiology
See this page for details of the causal pathogens. Some of the virus strains can infect swine, poultry and other farmed birds including quail and ratites. Isolated cases have also been noted in cattle, sheep and non-domestic ungulates. Some strains are potential agents of biowarfare or bioterrorism (Steele and Twenhafel, 2010).
Epidemiology
Distribution
Togaviral encephalitis in equids is largely confined to the Western Hemisphere. Venezuelan EEV can cause large outbreaks of disease over extensive geographical areas in both humans and horses. Spread of this virus into Central America has had disasterous consequences with epidemics as far north as Texas. Climatic conditions and interventions that support vector populations, such as irrigation, greatly influence the geographical spread of the disease. EEE has been recorded across the United States, but mostly in the Southeastern States. As its names suggests, WEE has a predilection for the Western states which have been subject to significant outbreaks in the past. A regional alteration in virulence has been proposed for the steep decline in clinical case numbers observed in this area. A lag phase of 2-5weeks is commonly observed between horse and human cases of WEE in a given locus. Both are dead-end hosts for the virus. A subtype of Western EEV, Highlands J virus, was isolated from the brain of a horse with encephalitis in Florida (Karabatsos et.al, 1988).
Transmission
Transfer is vector-mediated, primarily via mosquito salivary transfer. WEE and VEE may also be transmitted horse to horse through nasal secretions. This mode of transmission is less likely, despite the fact that high concentrations of VEE virus are found in ocular and nasal discharges from infected horses. The viraemic phase ends when nervous signs develop and is important for disease amplification. Amplification from horses is likely only with VEE virus, in association with a relatively high and potentially persistent viraemia. Similarly, zoonotic spread is unlikely for Eastern and Western equine encephalitis, but has been noted with VEE.
Seasonal Incidence
The disease is not directly contagious between horses and humans but occurs sporadically in both species from mid-summer to late autumn - during the height of the vector season. Case numbers peak in June to November in temperate climates. The vector season is longer in warmer climates, where the disease period is prolonged. Global warming may promote more outbreaks in historically colder climates.
Epidemics
Outbeak prediction to date has been inaccurate, implying that other, unidentified factors may be in operation. However, some epidemic requirements are beyond question. Adequate amounts of infective virus, sufficent vectors, infected sylvatic hosts and susceptible terminal hosts, and finally, appropriate reservoirs, are all crucial.
Pathogenesis
Upon entry to the host, viruses multiply in the muscle, enter the lymphatic circulation and localize in lymph nodes. In macrophages and neutrophils viral replication leads to shedding and significant clearance of viral particles. No further clinical signs develop if this clearance is successful. Erythrocyte and leukocyte absorption are used to circumvent the immune defences of the host. After incomplete elimination, residual virus infects endothelial cells and accumulates in highly vascular organs such as the liver and spleen. In these organs, viral replication produces circulating virus and a second viraemic period, typically associated with early clinical signs. Neuroinvasion and replication occurs within a week. An incubation period of 7-21days has been demonstrated after experimental infection with Eastern or Western EEV, but the incubtion is often shorter for EEE compared with that of WEE.
Signalment
Unvaccinated adult horses and other equids are at risk in areas with suitable vectors. Vaccinated horses can still develop the disease, particularly if they are young or old.
Clinical Signs
Worse in unvaccinated horses.
EEE and WEE
Following an incubation period of 7-21days, an initial pyrexia and mild depression are short-lived and often missed. The acute phase of the disease presents with mild to severe pyrexia, anorexia and stiffness, lasting up to 5 days. During this time, the horse is viraemic and capable of amplifying the disease. The fever may then fluctuate with neurological derangements appearing a few days post-infection. These changes indicate disease progression, which occurs more frequently with EEE (the most virulent of the three serotypes). Any of the following may be observed:
- conscious proprioceptive deficits
- propulsive walking
- depression
- somnolence
- aggression
- excitability
- restlessness
- hypersensitivity to sound and touch
Worsening neurological deficits may result in:
- head pressing
- head tilt
- circling
- apparent blindness
- facial and appendicular muscle fasciculations
- pharynx, larynx and tongue paralysis
- recumbency for 1-7 days followed by death
VEE
Clinical signs may resemble those of WEE and EEE or may vary:
- pyrexia peaks early and persists throughout the disease course
- mild pyrexia and leukopenia with endemic strains
- severe pyrexia and leukopenia with epidemic strains
- neurological signs around 4 days post-infection
- diarrhoea, severe depression, recumbency and death may precede neurological signs
- other signs: abortion, oral ulceration, pulmonary haemorrhage, epistaxis
Diagnosis
Presumptive based on epidemiology and clinical signs. Definitive diagnosis requires serological tests and/or post-mortem examination. Virus isolation can be performed from blood or spinal fluid samples
Laboratory Tests
Virus Identification
The virus is identified by complement fixation, immunofluorescence, PCR, ELISA, virus isolation or plaque reduction neutralisation tests.
- The Complement Fixation Test can be used to identify Eastern or Western EEV in infected mouse or chicken brains, cell culture fluid or amniotic-allantoic fluid
- Immunofluroescence: virus may be identified in brain tissue or cell culture using direct immunofluorescent staining.
- PCR: EEE and WEE viral RNA may also be detected by reverse-transcription PCR. EEE virus nucleic acid in mosquitoes and tissues has been identified by the polymerase chain reaction (PCR) using primers selected from the capsid gene (15). An alternate identification procedure is by hybridisation with an oligonucleotide probe. A reverse-transcription PCR method for detection of WEE RNA and alternative methods for EEE RNA detection have also been described (5, 7).
- ELISA can be used to detect virus in brain tissue. An antigen-capture ELISA, developed for EEE surveillance in mosquitoes, can be used where virus isolation and PCR facilities are unavailable (1).
Virus isolation Virus isolation is the most definitive method for diagnosis of EEE or WEE. EEE virus can usually be isolated from the brains of horses, unless more than 5 days have elapsed between the appearance of clinical signs and the death of the horse. EEE virus can frequently be isolated from brain tissue even in the presence of a high serum antibody titre. WEE virus is rarely isolated from tissues of infected horses. Brain is the tissue of choice for virus isolation, but the virus has been isolated from other tissues, such as the liver and spleen. It is recommended that a complete set of these tissues be collected in duplicate, one set for virus isolation and the other set in formalin for histopathological examination. Specimens for virus isolation should be sent refrigerated if they can be received in the laboratory within 48 hours of collection; otherwise, they should be frozen and sent with dry ice. Newborn mice, chicken embryos and a number of cell culture systems can be used for virus isolation. Virus may be isolated from the CSF of acutely infected horses
Serology
A combination of complement fixation (CF), haemagglutination inhibition (HAI) and cross-serum neutralization assays supports the acquisition of a positive diagnosis. Serological confirmation of EEE or WEE virus infection requires a four-fold or greater increase or decrease in antibody titre in paired serum samples collected 10-14 days apart. Most horses infected with EEE and WEE virus have a high antibody titre when clinical disease is observed. Horses infected with EEE or WEE virus usually have antibody titres in the acute stage of the disease. Consequently, a presumptive diagnosis can be made if an unvaccinated horse with appropriate clinical signs has antibody against only EEE or WEE virus. The detection of IgM antibody by the ELISA can also provide a presumptive diagnosis of acute infection (11). The plaque reduction neutralisation (PRN) test or, preferably, a combination of PRN and haemagglutination inhibition (HI) tests is the procedure most commonly used for the detection of antibody against EEE and WEE viruses. There are cross-reactions between antibody against EEE and WEE virus in the CF and HI tests. CF antibody against both EEE and WEE viruses appears later and does not persist; consequently, it is less useful for the serological diagnosis of disease.
A 4-fold increase in antibody (Ab) titre in convlescent sera is quoted for diagnosis but this test lacks sensitivity. The presence of viral Abs within 24hours of the initial viraemia typically precedes clinical signs. Ab titre increases sharply then deteriorates over 6 months. Samples taken when clinical signs appear are likely to miss the Ab peak and may thus demonstrate a decreasing titre. A single sample demonstrating an increased titre using HAI, CF and neutralizing Ab can provide a presumptive diagnosis. Maternal-derived Ab may interfere with diagnosis in foals. The serum half-life of colostral Ab in foals is around 20days.
a) Complement fixation
To avoid anticomplementary effects, sera should be separated from the blood as soon as possible. Positive and negative control sera should be used in the test.
b) Haemagglutination inhibition
Titres of 1/10 and 1/20 are suspect, and titres of 1/40 and above are positive.
c) Enzyme-linked immunosorbent assay
Viral-specific IgM to the surface glycoprotein of Venezuelan EEV may be detected by ELISA, from 3 days post-onset of clinical signs up to 21 days post-infection. The ELISA is useful in acute VEE infections where convalescent serum samples are unobtainable. The ELISA is performed by coating flat-bottomed plates with anti-equine IgM capture antibody (11). A test sample is considered to be positive if the absorbance of the test sample in wells containing virus antigen is at least twice the absorbance of negative control serum in wells containing virus antigen and at least twice the absorbance of the sample tested in parallel in wells containing control antigen.
d) Plaque reduction neutralisation
The PRN test is very specific and can be used to differentiate between EEE and WEE virus infections. The PRN test is performed in duck embryo fibroblast, Vero, or BHK-21 cell cultures. Endpoints are based on a 90% reduction in the number of plaques compared with the virus control flasks.
Clinical Pathology
Cerebrospinal fluid (CSF) samples demonstrate increased cellularity (50-400 mononuclear cells/µl) and protein concentration (100-200mg/dl).
Post-mortem findings
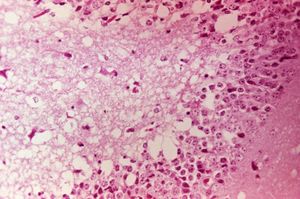
PRECAUTION: infective viral particles may be present in CNS and other tissues. Gross pathological lesions of the brain and spinal cord are rarely seen in horses, although traumatic ecchymotic haemorrhages and vascular congestion of the CNS may be evident. The extent of microscopic lesions is dictated by the severity of infection and duration of neurological involvement (Walton, 1981). Such lesions with or without immunohistochemistry may be diagnostic. The cerebral cortex, thalamus and hypothalamus are often severely affected. Mononuclear meningitis, neuronal degeneration, diffuse and focal gliosis and perivascular cuffing are also seen. Histological lesions of EEE are usually present throughout the CNS, with widespread and severe neutrophilic inflammation of the grey matter. Lesions caused by Western EEV infection are more focal and lymphocytic in nature. VEE cases often exhibit haemorrhage and liquefactive necrosis of the cerebral cortex, but lesions are not restricted to the CNS. In the pancreas, acinar cells atrophy and duct cells undergo hyperplasia. There may also be damage to the liver and heart.
Differential diagnoses
- Other togaviral encephalitides
- Trauma
- Hepatic encephalopathy
- Rabies
- Leukoencephalomalacia
- Bacterial meningoencephalitis
- Equine protozoal myeloencephalitis (EPM)
- Verminous encephalomyelitis
- West Nile Virus (WNV) infection
- Toxicosis
Treatment
No effective, specific treatment is available. Supportive management includes:
- NSAIDs (phenylbutazone, flunixin meglumine) to control pyrexia, inflammation and discomfort
- DMSO IV in a 20% solution to control inflamation, provide some analgesia and mild sedation
- Pentobarbital, diazepam IV, phenobarbital PO or phenytoin IV to control seizures
- Antibiotic therapy in cases with secondary bacterial infection
- Balanced fluid solutions IV or PO as necessary to correct hydration status
- Dietary supplementation (enteral, or parenteral if anorexia persists more than 48 hours)
- Laxatives to reduce the risk of impaction
- Protection of body regions susceptible to self-induced trauma and provision of deep bedding
- Sling support for recumbent horses
Prognosis
Comatose animals rarely survive. Survivors exhibit functional improvement over weeks to months, but complete recovery from neurological deficits is rare. Residual ataxia, depression and abnormal behaviour is often seen with EEE, but less so with WEE. The mortality rates for neurological equine viral encephalitis are reportedly:
- EEE 75-100%
- WEE 20-50%
- VEE 40-80%
It is generally assumed that infection does not provide protective immunity, however, protection for up to 2 years has been noted.
Control
Vaccination
Most vaccines are killed (produced in cell culture and inactivated with formalin) and elicit significant increases in Ab titre after 3 days. Protective titres last for 6-8 months. Some cross-protection is seen between the serotypes but not between Western and Eastern EEV. Monovalent, divalent and trivalent vaccines are available but the response to monovalent VEE vaccination is decreased in horses previously vaccinated against WEE and EEE. The current recommendation is to vaccinate susceptible horses annually in late spring or several months before the high risk season. Biannual or triannual vaccination should be employed in regions where the vector season is prolonged. Susceptible horses should also be vaccinated in the face of an outbreak. Mares should be vaccinated one month prior to foaling to boost colostral-derived Ab, which persists for 6-7 months. Although foals can be vaccinated at any time, early vaccination should be followed by boosters at 6 months and at one year. Vaccination does not interfere with the ELISA assay for VEE. PRECAUTION: human vaccination is recommended for vets in endemic areas.
Vector control
Responsible use of insecticides and repellents, elimination of standing water, and stable screening will all help to reduce viral transmission. Environmental application of insecticides may be useful in endemic areas or during an outbreak. Horses infected with Venezuelan EEV should be isolated for 3 weeks after complete recovery.
References
Bertone, J.J (2010) Viral Encephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine, Third Edition, Saunders, Chapter 12.
Manual of Diagnostic Tests and Vaccines for Terrestrial Animals http://www.oie.int/eng/normes/mmanual/A_00081.htm 4. Karabatsos N., Lewis A.L., Calisher C.H., Hunt A.R. & Roehrig J.T. (1988). Identification of Highland J virus from a Florida horse. Am. J. Trop. Med. Hyg., 39, 603-606. Walton T.E. (1981). Venezuelan, eastern, and western encephalomyelitis. In: Virus Diseases of Food Animals. A World Geography of Epidemiology and Control. Disease Monographs, Vol. 2, Gibbs E.P.J., ed. Academic Press, New York, USA, 587-625.
http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/viralenceph/index.htm
Vet Pathol. 2010 Jun 15. [Epub ahead of print] Review Paper: Pathology of Animal Models of Alphavirus Encephalitis. Steele KE, Twenhafel NA.
| Also known as: | Alphaviral encephalitis, Alphaviral encephalitides Eastern equine encephalitis, Eastern equine encephalomyelitis, EEE |