Bone marrow aspirate and biopsy
The most common indications for bone marrow evaluation are persistent cytopaenias (neutropaenia, thrombocytopenia, poorly regenerative anaemia) and the presence of immature haematopoietic cells in the circulation. The haematological abnormalities should not be readily explained by the history, clinical examination, current therapy or the biochemistry profile. For example, animals with severe renal disease may have a non-regenerative anaemia due to reduced erythropoietin production by the kidney. Under these circumstances bone marrow aspiration would not provide additional information. Bone marrow evaluation may be used to assess marrow
involvement in neoplastic conditions such as lymphoid or mast cell neoplasia and multiple myeloma. It can also be used to identify suspected infectious disease for example Leishmania.
Contraindications
There are few significant contraindications. Restraint, sedation andanaesthesia often are a greater risk than the marrow biopsy. Haemorrhage is a complication which might be expected in thrombocytopaenic animals, but in practice, significant bleeding rarely occurs, even in severely thrombocytopaenic animals. In some cases, digital pressure over the biopsy site may be needed to control haemorrhage. Post biopsy bleeding has been reported in animals with a monoclonal gammopathy. Iatrogenic marrow infection is possible, but the risk is minimal if the overlying skin has been properly prepared.
Bone marrow aspiration technique
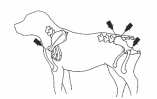
Bone marrow aspiration biopsies may be collected from the iliac crest, trochanteric fossa of the femur, proximal humerus and sternebrae of dogs and cats (Fig 1). The iliac crest in large dogs and the trochanteric fossa in small dogs and cats are most commonly used because they are most accessible and require least chemical restraint. The sternebrae and ribs should be avoided in small dogs and cats due to the risk of penetration of the thoracic cavity. In larger, obese or wellmuscled dogs the trochanteric fossa may not be accessible and in older dogs the cortical bone of the trochanteric fossa may be so dense that penetration of the marrow cavity is difficult. In these patients the humerus is a useful site. In small dogs and cats the small diameter of the bones makes the trochanteric fossa the site of preference.
Equipment
- A 16-18g, 1-2” bone marrow aspiration needle or a 13g 2-3½” aspiration/biopsy needle (Jamshidi needle). If a cat or small dog <5kg use a 16g needle. The use of a rotary battery powered device has been evaluated and may aid in the collection of better quality bone marrow samples
- A 10-20ml syringe
- Clean microscope slides
Trochanteric fossa method
- Place the animal in lateral recumbency
- Clip the hair for several inches around the expected site of needle penetration and prepare as for any aseptic surgical procedure
- Inject the skin at the puncture site with local anaesthetic and the periosteum at the expected site of penetration
- Make a small stab incision in the skin with a sterile scalpel blade
- Palpate the greater trochanter. Pass the biopsy needle medial to the greater trochanter with its long axis parallel to the long axis of the femur, until the needle reaches the trochanteric fossa
- When the bone is contacted, apply moderate pressure while rotating the needle clockwise and then anticlockwise. Decreased resistance is felt when the marrow cavity is entered
- Remove the stylet when the needle is in the marrow cavity
- Attach a 10-20ml syringe to the needle. Apply strong/rapid negative pressure and after a few drops of marrow have been collected release the negative pressure. Animals may appear uncomfortable as aspiration begins. Continued negative pressure or repeated aspiration in the same area will lead to excessive blood contamination of the marrow. If marrow is not aspirated at the first site, replace the stylet and reposition the needle by slightly advancing and rotating the needle, apply negative pressure. If there is still no marrow, continue the negative pressure as the needle is withdrawn slowly, either until marrow is aspirated or the bone is exited. If a sample cannot be aspirated then a core or incisional biopsy may be required
- As soon as marrow appears in the syringe, release the negative pressure, detach the syringe leaving the needle in the bone and replace the stylet
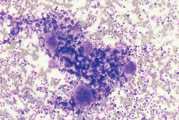
- Expel the sample onto a tilted microscope slide so that blood drains off. Marrow flecks tend to adhere to the slide. Place a second glass slide perpendicularly across the marrow flecks and then pull the slides smoothly apart in a horizontal plane dispersing the flecks (Fig 2). Beware of excessive pressure during spreading as this can cause disruption of cells
- If no marrow flecks appear to be present remove the stylet, reattach another syringe and repeat aspiration
- Rapidly air-dry the slides. Do not package the slides until dry as the sample quality will be
- affected
Iliac crest method
- Prepare the site aseptically, as described previously
- Make a small stab incision in the skin with a sterile scalpel blade
- The needle is positioned over the widened dorsal aspects of the iliac crest. When the bone is contacted, apply moderate pressure while rotating the needle clockwise and then anticlockwise. Decreased resistance may not be felt at this site when the marrow cavity is entered
- Remove the stylet and aspirate bone marrow as described previously
Humerus method
- Prepare the site aseptically
- Make a small stab incision in the skin with a sterile scalpel blade
- Insert the biopsy needle into the craniolateral humerus, below the tubercle and collect a sample as described previously
Core biopsy
Core biopsies can be collected from the iliac crest in large dogs and via a transverse approach to the wing of the ileum in small dogs, using a Jamshidi needle. The collection procedure is similar to that for marrow aspirate but the stylet is removed after the needle penetrates the bone marrow and the needle is then advanced a further 1cm with a rotating motion. The needle should be rotated in a ‘stirring’ action to dislodge the base of the core biopsy. The needle is withdrawn and the core pushed out by inserting a probe into the needle from the cutting edge. Impression smears are made by gently rolling the core onto clean glass slides and the remaining material is fixed in formal saline for histological examination. The use of a rotary battery-powered device has been evaluated and may aid in the collection of better quality bone marrow samples.
Please visit www.nwlabs.co.uk or see our current price list for more information