Neoplasia of Haematopoietic Bone Marrow, solid Lymphoid organs and Blood
Myeloid Neoplasia
Myeloid neoplasia (formerly termed myeloproliferative disease) refers to all leukaemias originating from (non-lymphoid) haematopoietic stem cells, called common myeloid progenitor cells, which may differentiate to produce granulocytic, monocytic, erythrocytic and megakaryocytic lineages. Myeloid neoplasms are subclassified as myelodysplastic syndromes, acute myeloid leukaemiasand myeloproliferative neoplasms (formerly chronic myeloproliferative disease).
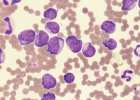
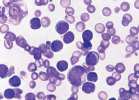
Haematopoietic neoplasia occurs when there is malignant transformation of a pluripotential stem cell or committed stem cell. In chronic myeloid neoplasia cells are differentiating or are mature; in acute myeloid leukaemia immature cells predominate in the blood and bone marrow. There is often a nonregenerative anaemia with variable WBC count and possibly thrombocytopaenia. There may be hepatosplenomegaly due to extramedullary haematopoiesis and/or neoplastic infiltration.
Neoplastic myeloid cells may be identified by their sequential differentiation into more mature cells in chronic forms of disease. However, blast cells which characterize acute myeloid leukaemias often lack identifiable characteristics. Flow cytometry is used in conjunction with a panel of cell surface markers (CD markers) to identify malignant cell populations in the blood or bone marrow. This is the recommended method for identification of atypical cell populations. A fresh EDTA sample is required.
Acute Myeloid Leukaemia (AML)
A classification system for AML has been adapted from the French-American-British system, established by the National Cancer Institute workshop for use in humans. AML is diagnosed when the percentage of non-lymphoid haematopoietic blast cells in the bone marrow equals or is >30% of all nucleated cells (ANCs) excluding lymphocytes, plasma cells, mast cells and macrophages. [This percentage has been decreased to 20% for humans in the most recent WHO classification scheme and this may be adopted in veterinary medicine in the future]. Blast cells may also be quantified according to their percentage of all nonerythroid cells (NECs), determined by subtracting the nucleated erythroid cells from the ANC count. Dyshaematopoiesis is usually present. If the percentage of blast cells is <30% and dysplastic changes are present, the diagnosis is myelodysplastic syndrome (MDS).
AML may be subtyped by flow cytometry using lineage specific antigens, by immunohistochemistry, immunocytochemistry or cell morphology in cases showing differentiation.
Whilst dyshaematopoiesis with disorderly maturation suggests neoplasia it can occur during repopulation of bone marrow after destruction (Parvovirus, toxic effect, radiation). With repopulation, although initially immature cells predominate mimicking leukaemia, progressive maturation leads to an orderly population. Therefore, in certain cases, sequential monitoring of the bone marrow may be necessary.
Subtypes of AML
Acute undifferentiated leukaemia (AUL). There are large numbers of blast cells which cannot beidentified with certainty. The blast cells have broad cytoplasmic pseudopods often with magenta staining cytoplasmic granules.
AML-M1. Myeloblastic leukaemia without maturation, >90% of nonerythroid cells (NECs) in the bone marrow are myeloblasts.
AML-M2. Myeloblastic leukaemia with maturation, 30-90% of NECs are myeloblasts. 10% or more of NECs are differentiated granulocytes.
AML-M3. Promyelocytic leukaemia,reported in humans and pigs. Proliferating cells have a few azurophilic granules, cytochemistry suggests neutrophilic lineage.
AML-M4. Acute myelomonocytic leukaemia, numbers of myeloblasts and monoblasts combined equal or exceed 30% of ANCs; differentiated granulocytes and monocytes each represent 20% or more of NECs. This is the most common AML seen in dogs, cats and horses.
AML-M5. Acute monocytic leukaemia, monoblasts are increased, myeloblasts are not. Monoblasts and promonocytes account for >80% of NECs. Monocytes in the blood may be increased and show abnormal morphology.
AML-M6. Erythroleukaemia has a dual cell lineage with production of both erythroblasts and myeloblasts. >50% of ANCs are erythroid precursors and >30% of NECs are blasts (myeloblasts, monoblasts and megakaryoblasts). The M:E ratio is <1.
AML-M7. Megakaryocytic leukaemia, >30% of nucleated cells in the bone marrow are megakaryoblasts. This is often associated with myelofibrosis, impeding aspiration of bone marrow. A severe nonregenerative anaemia is consistently present in dogs.
Myelodysplastic Syndrome (MDS)
Myelodysplasia is the term used when more than 10% of cells in one or more bone marrow lines are dysplastic. MDS is characterized by impaired survival of neoplastic cells and impaired differentiation. There are peripheral cytopaenias (typically nonregenerative anaemia and thrombocytopaenia) with atypical cells in circulation. Feline retroviral infections can produce MDS. The disease may progress to AML after a variable period of time.
Myeloproliferative neoplasms (MPN)
MPNs (formerly termed chronic myeloproliferative disease) are characterised by neoplastic proliferations of haematopoietic cells resulting in high numbers of differentiated cells in circulation. Blast cells represent <30% ANCs.
Subtypes of MPN
Chronic myeloid leukaemia (CML). Rare and occurs predominantly in dogs. It presents with a marked leucocytosis with a left shift. Numbers of monocytes, eosinophils and basophils may be increased. Circulating blast cells are absent or rare. In the bone marrow there is granulocytic hyperplasia with an increased proportion of immature cells, however, myeloblasts represent <30% of ANCs. Diagnosis is often based on finding a marked persistent leucocytosis having excluded a severe inflammatory leukemoid reaction (associated with peritonitis, pyometra, hepatic abscess etc) or a paraneoplastic syndrome with production of granulocyte or granulocyte-macrophage colony stimulating factor (renal carcinoma, rectal adenoma, fibrosarcoma and other tumours). There may be lymphadenopathy due to proliferation of myeloid cells in the lymph nodes (occasionally extramedullary haematopoiesis can be seen in lymph nodes). Neoplastic cells may also proliferate in the liver.
Chronic granulocytic leukaemia, chronic myelomonocytic leukaemia and chronic monocytic leukaemia may be considered variants of CML.
Chronic eosinophilic leukaemia. A variant of CML with marked eosinophilia in bone marrow and blood, with variable eosinophil maturation. Differentiation of eosinophilic leukaemia and hypereosinophilic syndrome in cats can be difficult; the latter shows hypereosinophilia with frequent involvement of the GIT. Eosinophilic left shifts in the peripheral blood with organ infiltration may be more common in eosinophilic leukaemia.
Chronic basophilic leukaemia. A variant of CML whereby basophils predominate in the bone marrow and blood. This has been reported rarely in dogs. Immature basophils in circulation must be differentiated from mast cells in cases of mastocytaemia. Basophils have an indentedlobulated nucleus (depending on the degree of maturation) whereas mast cells have a round nucleus.
Primary erythrocytosis (polycythaemia vera). The neoplastic production of anucleate, mature erythrocytes independent of erythropoietin levels. The bone marrow shows erythroid hyperplasia with orderly maturation of cells. The PCV is markedly increased (up to 81%). Diagnosis requires demonstration of absolute increased red cell mass with normal Pa02 and decreased serum erythropoietin.
Essential thrombocythaemia. Rare but has been reported in dogs and cats. Megakaryocytes are numerous in the bone marrow. In the blood, platelet counts often exceed 1,000 x 109/l and may show abnormal morphology (intense granulation and giant platelets). Physiologic thrombocytosis due to iron deficiency anaemia, recent haemorrhage, chronic inflammatory disease and splenectomy must be ruled out.
Lymphoid Neoplasms
Lymphoma is characterised by the neoplastic (clonal) proliferation of lymphoid cells forming solid tumours out with the bone marrow. Lymphoid leukaemia describes a neoplastic condition of lymphocytes within the bone marrow and/or blood which is not associated with a solid tumour and may be acute (ALL) or chronic (CLL) depending on the degree of cell maturity. When neoplastic lymphocytes are present in the peripheral circulation of a patient with lymphoma (late clinical stage disease) this is described as leukaemic lymphoma (also referred to as lymphomawith overspill, lymphosarcoma cell leukaemia).
Lymphoma. May be classified according to anatomic site (multicentric, mediastinal, alimentary, cutaneous), lymphoid morphology, site arising within the lymph node or a combination of these. In dogs multicentric, alimentary, cutaneous and mediastinal lymphoma are reported with decreasing frequency. In cats alimentary lymphoma in older cats is most frequently reported, with multicentric, mediastinal and extranodal forms seen less commonly. This is in contrast to previous reports, prior to the widespread use of FeLV vaccine, where thymic lymphoma in young cats was most common.
Diagnosis is by FNA or biopsy of affected lymphoid tissue. In dogs, flow cytometry, PARR testing or immunocytochemistry on fixed tissue can be used for immunophenotyping as this has prognostic implications (although B is not always bad and T is not always terrible); zonal lymphomas of either B or T cell origin may progress slowly.
In cats lymph node FNA is generally not sufficient to confirm lymphoma due to the difficulties encountered when trying to differentiate lymphoma from the range of benign hyperplastic syndromes unique to this species. Biopsy with histopathological evaluation is required for a definitive diagnosis. Immunohistochemistry can be used to determine phenotype but this has not been reliably proved to predict outcome.
Acute lymphoblastic leukaemia (ALL). Describes the condition where lymphoblasts and prolymphocytes are present in the bone marrow and/or peripheral circulation. There may be lymphocytosis, frequently with neutropaenia, thrombocytopaenia and a moderate-severe nonregenerative anaemia. Clinical progression is rapid due to myelophthisic disease. Diagnosis requires bone marrow cytologic assessment; lymphoblasts may be difficult to differentiate from other blast cells, cytochemical techniques can be used, however, flow cytometry is the method of choice. Most cases of feline ALL have a T cell phenotype and are FeLV (or FIV) positive. In dogs neoplastic cells in ALL may have a B cell, T cell, NK or null cell phenotype.
Chronic lymphocytic leukaemia (CLL). Characterised by large numbers of lymphocytes in the peripheral blood and bone marrow. Lymphocytosis (6-200 x109/l) is consistently present with normal lymphocyte morphology.
There may be a mild-moderate anaemia and thrombocytopaenia (neutropaenia is rare), lymphadenopathy and hepatosplenomegaly. This type of leukaemia is seen in older animals and may be an incidental finding when diagnosed. T cell CLL is more common than B cell CLL in dogs and cats. The majority of cats with CLL are FeLV negative. The less common, canine B cell CLL, may have an accompanying monoclonal gammopathy of the IgM type.
There is a high lymphocyte count with predominantly small lymphocytes. Occasional larger blast cells are present and these may become more numerous as a blast crisis develops.