Difference between revisions of "Hepatic Stellate Cells"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
[[Image:Hepatic stellate cells figure.jpg|thumb|right|300px|'''Hepatic Stellate Cells, WikiMedia Commons, 2007.'''<br /> | [[Image:Hepatic stellate cells figure.jpg|thumb|right|300px|'''Hepatic Stellate Cells, WikiMedia Commons, 2007.'''<br /> | ||
Schematic presentation of hepatic stellate cells (HSC) located in the vicinity of adjacent hepatocytes (PC) beneath the sinusoidal endothelial cells (EC). S – liver sinusoids; KC – Kupffer cells. Down left shows cultured HSC at light-microscopy, whereas at down right electron microscopy (EM) illustrates numerous fat vacuoles (L) in a HSC, in which retinoids are stored.]] | Schematic presentation of hepatic stellate cells (HSC) located in the vicinity of adjacent hepatocytes (PC) beneath the sinusoidal endothelial cells (EC). S – liver sinusoids; KC – Kupffer cells. Down left shows cultured HSC at light-microscopy, whereas at down right electron microscopy (EM) illustrates numerous fat vacuoles (L) in a HSC, in which retinoids are stored.]] | ||
| Line 8: | Line 7: | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
| − | HSCs are derived from the neural crest and therefore express synaptophysin, GFAP, neural cell adhesion molecule, nestin, neurotrophins, and their receptors. HSCs are located between the parenchymal cell plates and endothelial linings, with long cytoplasmic filaments that connect them to endotheial cells and parenchymal cells. As indicated above HSCs have four main functions including retinoid storage and homeostasis, remodelling of ECM by production of ECM components and MMPs, production of growth factors and cytokines (likely have a role in hepatocyte regeneration following injury) and the contraction and dilation of the sinusoidal lumen in response to endothelin, angiotensin, thromboxane or prostaglandins. HSCs exhibit 2 physiological states. The first is quiescent where they can be seen to contain cytoplasmic lipid droplets containing retinyl esters and long cytoplasmic processes with fine branches. The second state is activated/transdifferentiated where they lack lipid droplets, display proliferative and fibrongenic myofibroblast-like phenotype (activated state expresses alpha smooth muscle actin and desmin). These states are regulated by paracrine and autocrine loops of growth factors (TGF-beta-1). | + | HSCs are derived from the neural crest and therefore express synaptophysin, GFAP, neural cell adhesion molecule, nestin, neurotrophins, and their receptors. HSCs are located between the parenchymal cell plates and endothelial linings, with long cytoplasmic filaments that connect them to endotheial cells and parenchymal cells (see image). As indicated above HSCs have four main functions including retinoid storage and homeostasis, remodelling of ECM by production of ECM components and MMPs, production of growth factors and cytokines (likely have a role in hepatocyte regeneration following injury) and the contraction and dilation of the sinusoidal lumen in response to endothelin, angiotensin, thromboxane or prostaglandins. HSCs exhibit 2 physiological states. The first is quiescent where they can be seen to contain cytoplasmic lipid droplets containing retinyl esters and long cytoplasmic processes with fine branches. The second state is activated/transdifferentiated where they lack lipid droplets, display proliferative and fibrongenic myofibroblast-like phenotype (activated state expresses alpha smooth muscle actin and desmin). These states are regulated by paracrine and autocrine loops of growth factors (TGF-beta-1). |
<br /> | <br /> | ||
<br /> | <br /> | ||
| − | |||
[[Category:Liver and Gall Bladder - Anatomy & Physiology]] | [[Category:Liver and Gall Bladder - Anatomy & Physiology]] | ||
| − | [[Category:A&P | + | [[Category:To Do - A&P]] |
Revision as of 18:45, 19 January 2011
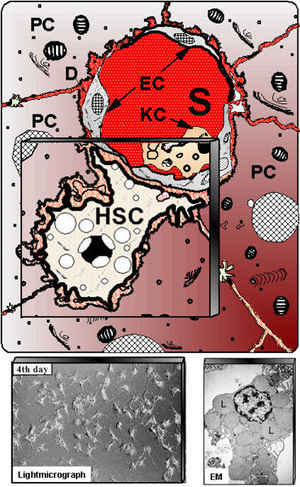
Schematic presentation of hepatic stellate cells (HSC) located in the vicinity of adjacent hepatocytes (PC) beneath the sinusoidal endothelial cells (EC). S – liver sinusoids; KC – Kupffer cells. Down left shows cultured HSC at light-microscopy, whereas at down right electron microscopy (EM) illustrates numerous fat vacuoles (L) in a HSC, in which retinoids are stored.
Hepatic stellate cells (HSC) can also be referred to as vitamin A-storing cells, lipocytes, interstitial cells, fat-storing cells and Ito cells. HSC exist in the space between parenchymal cells and sinusoidal endothelial cells of the hepatic lobule and store 80% of retinoids in the whole body as retinyl palmitate in lipid droplets in the cytoplasm. In physiological conditions, these cells play pivotal roles in the regulation of retinoid homeostasis; they express specific receptors for retinol-binding protein (RBP), a binding protein specific for retinol, on their cell surface, and take up the complex of retinol and RBP by receptor-mediated endocytosis. HSCs in arctic animals such as polar bears and arctic foxes store 20–100 times the levels of retinoids found in humans or rats.
In pathological conditions such as liver fibrosis, HSCs lose retinoids, and synthesize a large amount of extracellular matrix (ECM) components including collagen, proteoglycan, and adhesive glycoproteins. The morphology of these cells also changes from the star-shaped stellate cells to that of fibroblasts or myofibroblasts. The three-dimensional structure of ECM components was found to regulate reversibly the morphology, proliferation, and functions of HSCs. Molecular mechanisms in the reversible regulation of the cells by ECM imply cell-surface integrin binding to ECM components, followed by signal transduction processes and then cytoskeleton assembly. HSCs also exist in extrahepatic organs such as pancreas, lung, kidney, and intestine. Hepatic and extrahepatic stellate cells form the stellate cell system.
HSCs are derived from the neural crest and therefore express synaptophysin, GFAP, neural cell adhesion molecule, nestin, neurotrophins, and their receptors. HSCs are located between the parenchymal cell plates and endothelial linings, with long cytoplasmic filaments that connect them to endotheial cells and parenchymal cells (see image). As indicated above HSCs have four main functions including retinoid storage and homeostasis, remodelling of ECM by production of ECM components and MMPs, production of growth factors and cytokines (likely have a role in hepatocyte regeneration following injury) and the contraction and dilation of the sinusoidal lumen in response to endothelin, angiotensin, thromboxane or prostaglandins. HSCs exhibit 2 physiological states. The first is quiescent where they can be seen to contain cytoplasmic lipid droplets containing retinyl esters and long cytoplasmic processes with fine branches. The second state is activated/transdifferentiated where they lack lipid droplets, display proliferative and fibrongenic myofibroblast-like phenotype (activated state expresses alpha smooth muscle actin and desmin). These states are regulated by paracrine and autocrine loops of growth factors (TGF-beta-1).