Difference between revisions of "Q Fever"
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
{{Taxobox | {{Taxobox | ||
|name = ''Coxiella burnetii'' | |name = ''Coxiella burnetii'' | ||
| Line 17: | Line 16: | ||
|species = ''C. burnetii'' | |species = ''C. burnetii'' | ||
}} | }} | ||
| − | |||
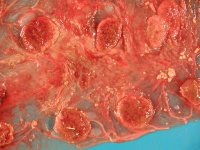
| − | + | [[File:Q Fever.jpg|thumb|200px|right|Goat placenta from Q fever abortion. Note the thickened intracotyledonary areas and tan exudate. The cotyledons have tan necrotic margins and congested, red centres. Copyright CFSPH Iowa State.]] | |
| + | Also Known As – '''''Abattoir fever – Australian Q Fever – Coxiellosis – Derrick-Burnet Disease – Balkan grippe – Nine mile fever – Quadrilateral fever – Pneumorickettsiosis – Hibernovernal bronchopneumonia''''' | ||
| + | |||
| + | Caused By – ''Coxiella burnetii'' | ||
==Introduction== | ==Introduction== | ||
| − | Q fever is caused by the '''gram negative, intracellular [[Rickettsiales |rickettsial]] [[Bacteria |bacterium | + | Q fever is caused by the '''gram negative, intracellular [[Rickettsiales | rickettsial]] [[Bacteria | bacterium]'''], ''Coxiella burnetii''. It causes '''respiratory''' and '''reproductive''' disease in animals and is an important '''zoonosis'''. The organism targets the '''[[Macrophages | macrophages]]''' of the host. |
It is a highly '''infectious''' disease affecting a wide range of species, but most importantly, '''humans''' and '''livestock'''. Wild animals are the natural reservoir for infection. | It is a highly '''infectious''' disease affecting a wide range of species, but most importantly, '''humans''' and '''livestock'''. Wild animals are the natural reservoir for infection. | ||
| Line 29: | Line 30: | ||
This disease is '''notifiable''' to the World Organisation for Animal Health [http://www.oie.int/ (OIE)] | This disease is '''notifiable''' to the World Organisation for Animal Health [http://www.oie.int/ (OIE)] | ||
| − | |||
==Distribution== | ==Distribution== | ||
Worldwide except for New Zealand. | Worldwide except for New Zealand. | ||
| − | ''C. burnetii'' is transmitted by many species of '''[[Ticks |tick]]''', including | + | ''C. burnetii'' is transmitted by many species of '''[[Ticks | tick]]''', including ''Amblyomma spp, Ixodes spp.'' and ''Dermacentor spp''. it has two interacting cycles of development involving small and large cell variants. Both are infectious. |
| − | + | Organisms can survive for '''long periods outside of a host'''; >586 days in tick faeces and months or years in the environment. | |
Most humans acquire the disease by '''inhalation'''. Cats, rabbits and birds along with other species may play roles in transmission to humans, on top of the main ruminant hosts. Humans rarely acquire the first, ectoparasite dependent cycle of disease, almost always being infected from domestic animals. | Most humans acquire the disease by '''inhalation'''. Cats, rabbits and birds along with other species may play roles in transmission to humans, on top of the main ruminant hosts. Humans rarely acquire the first, ectoparasite dependent cycle of disease, almost always being infected from domestic animals. | ||
| Line 59: | Line 59: | ||
==Diagnosis== | ==Diagnosis== | ||
| − | On gross examination, the [[Placenta - Anatomy & Physiology |'''placenta''']] of Q fever abortions is '''red-brown''' with '''light brown/tan exudates''' and soft, necrotic cotyledons. The aborted foetus may show '''hepatomegaly, subcutaneous oedema''' and reddish fluid accumulation. | + | On gross examination, the [[Placenta - Anatomy & Physiology | '''placenta''']] of Q fever abortions is '''red-brown''' with '''light brown/tan exudates''' and soft, necrotic cotyledons. The aborted foetus may show '''hepatomegaly, subcutaneous oedema''' and reddish fluid accumulation. |
| − | On histopathology, the | + | On histopathology, the placents is infiltrated by '''mononuclear cells''' and chorionic trophoblasts are necrotic. The exudates contains fibrin and neutrophils. The [[Liver - Anatomy & Physiology | liver]] displays histological vascular endothelial proliferation and diffuse leucocytic inflammation. '''Lymph follicles''' in the '''spleen''' and bronchial lymph nodes may be hyperplastic. Renal epithelial cells show vascular dystrophy and proliferation of fibroblasts. |
| − | ''C. burnetii'' can be confirmed by visualisation of '''smears''' from placenta, lung, liver and | + | ''C. burnetii'' can be confirmed by visualisation of '''smears''' from placenta, lung, liver and abomasums from the foetus or vaginal discharge, with '''Gimenez''' staining. |
| − | '''Serological''' antibody detection via Phase I antibodies indicate acute disease whereas Phase II are suggestive of prolonged, chronic infection. There are many methods of detecting antibodies including '''microagglutination, [[ELISA testing |ELISA]], | + | '''Serological''' antibody detection via Phase I antibodies indicate acute disease whereas Phase II are suggestive of prolonged, chronic infection. There are many methods of detecting antibodies including '''microagglutination, [[ELISA testing | ELISA]], IFAT''', Radioimmunoassays, Complement fixation and Western blot. |
| − | PCR is available from a range of clinical samples including milk and vaginal swabs and blood. | + | PCR is available from a range of flood and clinical samples including milk and vaginal swabs and blood. |
On ''post-mortem examination''', '''granulomatous lesions''' most commonly involve the lungs, liver and bone marrow. Typical “doughnut granulomas” form in the liver in acute cases. The liver is '''oedematous''' and fragile and spleen enlarged and hyperaemic. Consolidation, '''alveolar exudates''' and interstitial inflammatory exudates containing predominantly macrophages are consequences of the respiratory manifestation of disease. | On ''post-mortem examination''', '''granulomatous lesions''' most commonly involve the lungs, liver and bone marrow. Typical “doughnut granulomas” form in the liver in acute cases. The liver is '''oedematous''' and fragile and spleen enlarged and hyperaemic. Consolidation, '''alveolar exudates''' and interstitial inflammatory exudates containing predominantly macrophages are consequences of the respiratory manifestation of disease. | ||
| Line 77: | Line 77: | ||
At least 18 months of therapy is required for endocarditis. | At least 18 months of therapy is required for endocarditis. | ||
| − | |||
==Control== | ==Control== | ||
Reducing '''environmental contamination''' is part of control of Q fever in humans. The pasteurisation of milk and good hygiene measures are also very valuable. | Reducing '''environmental contamination''' is part of control of Q fever in humans. The pasteurisation of milk and good hygiene measures are also very valuable. | ||
| Line 83: | Line 82: | ||
'''Vaccination''' is also available for '''high risk individuals''' such as veterinarians and their staff, laboratory personnel and abattoir workers. These are based upon whole cell Phase I types of ''C. burnetii''. Safe use of vaccines requires '''screening''' by skin tests, serology or lymphocyte proliferation assays. | '''Vaccination''' is also available for '''high risk individuals''' such as veterinarians and their staff, laboratory personnel and abattoir workers. These are based upon whole cell Phase I types of ''C. burnetii''. Safe use of vaccines requires '''screening''' by skin tests, serology or lymphocyte proliferation assays. | ||
| − | The dangers of vaccinating pre-infected animals means that widespread vaccination of domestic | + | The dangers of vaccinating pre-infected animals means that widespread vaccination of domestic stick is not currently used. |
| + | {{Learning | ||
| + | |literature search = [http://www.cabdirect.org/search.html?q=title%3A+%28Q+fever%29/ Q Fever Publications] | ||
| − | |||
|flashcards = [[Q Fever Flashcards]] | |flashcards = [[Q Fever Flashcards]] | ||
}} | }} | ||
| − | |||
==References== | ==References== | ||
<references/> | <references/> | ||
| − | + | Animal Health & Production Compendium, '''Coxiella burnetii datasheet''', accessed 08/06/2011 @ http://www.cabi.org/ahpc/ | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Animal Health & Production Compendium, '''Q Fever datasheet''', accessed 08/06/2011 @ http://www.cabi.org/ahpc/ | |
| − | [[Category: | + | [[Category:To Do - CABI review]] |
| − | [[Category: | + | [[Category:Rickettsiales]][[Category:Sheep Bacteria]][[Category:Goat Bacteria]][[Category:Cattle Bacteria]] |
Revision as of 12:04, 15 June 2011
| Coxiella burnetii | |
|---|---|
| Phylum | Proteobacteria |
| Class | Zymobacteria |
| Sub-class | Alphaproteobacteria |
| Order | Legionellales |
| Family | Coxiellaceae |
| Genus | Coxiella |
| Species | C. burnetii |
Also Known As – Abattoir fever – Australian Q Fever – Coxiellosis – Derrick-Burnet Disease – Balkan grippe – Nine mile fever – Quadrilateral fever – Pneumorickettsiosis – Hibernovernal bronchopneumonia
Caused By – Coxiella burnetii
Introduction
Q fever is caused by the gram negative, intracellular rickettsial [[Bacteria | bacterium]], Coxiella burnetii. It causes respiratory and reproductive disease in animals and is an important zoonosis. The organism targets the macrophages of the host.
It is a highly infectious disease affecting a wide range of species, but most importantly, humans and livestock. Wild animals are the natural reservoir for infection.
Q fever in humans manifests as acute, severe flu-like illness, particularly in abattoir and farm workers.
This disease is notifiable to the World Organisation for Animal Health (OIE)
Distribution
Worldwide except for New Zealand.
C. burnetii is transmitted by many species of tick, including Amblyomma spp, Ixodes spp. and Dermacentor spp. it has two interacting cycles of development involving small and large cell variants. Both are infectious.
Organisms can survive for long periods outside of a host; >586 days in tick faeces and months or years in the environment.
Most humans acquire the disease by inhalation. Cats, rabbits and birds along with other species may play roles in transmission to humans, on top of the main ruminant hosts. Humans rarely acquire the first, ectoparasite dependent cycle of disease, almost always being infected from domestic animals.
Sexual transmission of Q fever has also been reported.
Signalment
Cattle, goats and sheep are the primary domestic reservoir for Q fever.
Infected animals shed the pathogen in their urine, faeces, milk and birth materials. Infected cattle may become carriers of infection, with the agent localised in the mammary glands.
In Europe, Q fever cases are more frequent in Spring and Summer, thought to be due to the lambing season.
5/6 human cases of Q fever are in adult males between 20 and 50 years old.
Clinical Signs
Chronic Q fever is characterised by pneumonia, abortion, rhinitis and poor offspring viability in animals. Abortion usually occurs in the third trimester of gestation and may occur in storms over 2-4 weeks involving up to 50% of the flock/herd.
Anorexia, depression and fever usually accompany respiratory and reproductive disease.
In man, it causes severe headaches, chills, remittent fever, cough, photosensitivity, fatigue and malaise. Pneumonia and hepatitis may occur in acute disease, while chronic infection often causes endocarditis. Existing heart disease or valvular grafts etc are important predisposing factors in people. Q fever can cause abortion and premature birth in pregnant women. Approximately ¼ deveop pronounced respiratory signs and pneumonia.
Diagnosis
On gross examination, the placenta of Q fever abortions is red-brown with light brown/tan exudates and soft, necrotic cotyledons. The aborted foetus may show hepatomegaly, subcutaneous oedema and reddish fluid accumulation.
On histopathology, the placents is infiltrated by mononuclear cells and chorionic trophoblasts are necrotic. The exudates contains fibrin and neutrophils. The liver displays histological vascular endothelial proliferation and diffuse leucocytic inflammation. Lymph follicles in the spleen and bronchial lymph nodes may be hyperplastic. Renal epithelial cells show vascular dystrophy and proliferation of fibroblasts.
C. burnetii can be confirmed by visualisation of smears from placenta, lung, liver and abomasums from the foetus or vaginal discharge, with Gimenez staining.
Serological antibody detection via Phase I antibodies indicate acute disease whereas Phase II are suggestive of prolonged, chronic infection. There are many methods of detecting antibodies including microagglutination, ELISA, IFAT, Radioimmunoassays, Complement fixation and Western blot.
PCR is available from a range of flood and clinical samples including milk and vaginal swabs and blood.
On post-mortem examination', granulomatous lesions most commonly involve the lungs, liver and bone marrow. Typical “doughnut granulomas” form in the liver in acute cases. The liver is oedematous and fragile and spleen enlarged and hyperaemic. Consolidation, alveolar exudates and interstitial inflammatory exudates containing predominantly macrophages are consequences of the respiratory manifestation of disease.
Treatment
Doxycycline or a fluoroquinolone are the first line treatments for acute Q fever. Treatment is required for 2-3 weeks minimum.
In chronic infection, a combination of doxycycline and hydroxychloroquine is preferred.
At least 18 months of therapy is required for endocarditis.
Control
Reducing environmental contamination is part of control of Q fever in humans. The pasteurisation of milk and good hygiene measures are also very valuable.
Vaccination is also available for high risk individuals such as veterinarians and their staff, laboratory personnel and abattoir workers. These are based upon whole cell Phase I types of C. burnetii. Safe use of vaccines requires screening by skin tests, serology or lymphocyte proliferation assays.
The dangers of vaccinating pre-infected animals means that widespread vaccination of domestic stick is not currently used.
| Q Fever Learning Resources | |
|---|---|
 Test your knowledge using flashcard type questions |
Q Fever Flashcards |
 Search for recent publications via CAB Abstract (CABI log in required) |
Q Fever Publications |
References
Animal Health & Production Compendium, Coxiella burnetii datasheet, accessed 08/06/2011 @ http://www.cabi.org/ahpc/
Animal Health & Production Compendium, Q Fever datasheet, accessed 08/06/2011 @ http://www.cabi.org/ahpc/