Difference between revisions of "Bovine Leukaemia Virus"
| (27 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Also Known As: '''''Enzootic Bovine Leucosis — Bovine Leukosis — EBL — BLV — BoLV — Bovine Leukemia — Lymphosarcoma — [[Sporadic Bovine Leukosis]] — Bovine Malignant Lymphoma''''' | |
| + | |||
| + | ==Introduction== | ||
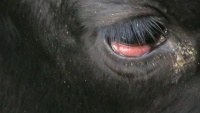
[[File:BLV.jpeg|thumb|200px|right|Conjunctival prolapse in cow with bovine viral leucosis (sourced from Wikimedia Commons)]] | [[File:BLV.jpeg|thumb|200px|right|Conjunctival prolapse in cow with bovine viral leucosis (sourced from Wikimedia Commons)]] | ||
| − | '''' | + | Bovine leukaemia virus is a [[:Category:Retroviridae|'''retrovirus''']] causing two specific diseases: '''Bovine lymphosarcoma''' and '''Persistent lymphocytosis'''. |
| − | + | Bovine lymphosarcoma is fatal while persistent lymphocytosis is not usually so. However, those affected with lymphosarcoma may or may not have been through the persistent lymphocytosis stage <ref>Radostitis et al (2007). Veterinary Medicine 10th Edition, Sauders Elsevier, Chapter 21</ref>. | |
| − | |||
| − | |||
| − | Bovine lymphosarcoma is fatal while persistent lymphocytosis is not usually so. | ||
Bovine leukosis is not transmissible to humans. | Bovine leukosis is not transmissible to humans. | ||
| − | This disease is notifiable to the World Organisation for Animal Health ([http://www.oie.int/ OIE]) | + | This disease is '''notifiable''' to the World Organisation for Animal Health ([http://www.oie.int/ OIE]) |
| − | =Signalment= | + | ==Signalment== |
| − | Cattle are thought to be the only species naturally susceptible, and prevalence rates are higher in dairy breeds than beef. | + | Cattle are thought to be the only species naturally susceptible, and prevalence rates are higher in '''dairy breeds''' than beef. |
| − | The majority of infected animals are more than 2 years of age, with younger animals developing sporadic bovine leukosis which is thought to be unrelated | + | The majority of infected animals are more than 2 years of age, with younger animals developing [[Sporadic Bovine Leukosis|sporadic bovine leukosis]] which is thought to be unrelated. |
Sheep are susceptible to experimental infection which appears more pathogenic in this species. | Sheep are susceptible to experimental infection which appears more pathogenic in this species. | ||
| Line 20: | Line 19: | ||
Manifestation of the fatal neoplastic lymphosarcoma form of disease also appears better represented in dairy cattle. | Manifestation of the fatal neoplastic lymphosarcoma form of disease also appears better represented in dairy cattle. | ||
| − | + | ==Distribution== | |
| + | BLV is globally distributed, but prevalence widely varies. The UK is currently free of infection evidenced by government funded bulk milk antibody surveillance and investigations of tumours found in live or dead animals <ref> DEFRA (2010). http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ebl/index.htm. Accessed 08/07/2012</ref>. | ||
| − | + | While 89% dairy operations in the USA showed seropositive results and 43.5% of all USA dairy cattle were seropositive <ref> USDA/APHIS/Veterinary Services, (1997). High prevalence of BLV in US dairy herds. Fort Collins, CO, USA: USDA: APHIS:VS Centers For Epidemiology and Animal Health.</ref>, seroprevalence in the EU rarely exceeds 0.5-1.5%. <ref>Lorenz, R. J., Straub, O. C. (1987) The epidemiology of enzootic bovine leukosis. In: Burny A, Mammerick, M, eds. Enzootic bovine leukosis and bovine leukemia virus. Boston, USA: Martinus Nijhoff Publishing, 51-68</ref> | |
| − | BLV | ||
| − | + | ==Transmission== | |
| − | + | Transmission of bovine leukaemia virus is mainly '''horizontal'''. '''Iatrogenic procedures''' are an important route as they facilitate transfer of '''contaminated blood''' between individuals, e.g. vaccinations, dehorning, rectal examinations and surgical procedures. Natural transmission by direct contact requires contact with infected blood, exudates or tissues which enter through broken skin. | |
| − | + | Vertical transmission is most likely from colostrum/milk and is less important, although it does provide a method for control. | |
| − | |||
| − | |||
| − | Transmission of bovine leukaemia virus is mainly horizontal. Iatrogenic procedures are an important route as they facilitate transfer of contaminated blood between individuals, e.g. vaccinations, dehorning, rectal examinations and surgical procedures. Natural transmission by direct contact requires contact with infected blood, exudates or tissues which enter through broken skin. | ||
| − | Vertical transmission is most likely from | ||
| + | ==Clinical Signs== | ||
===Lymphosarcoma=== | ===Lymphosarcoma=== | ||
| − | The most common presentation is that of enlarged lymph nodes which may cause multiple palpable swellings in the pharynx, flanks and limbs. | + | The most common presentation is that of '''enlarged lymph nodes''' which may cause multiple palpable swellings in the pharynx, flanks and limbs. This is often an early indicator of disease. |
| − | This is often an early indicator of disease. | ||
| − | 5-20% cases are peracute and present as sudden death. This | + | 5-20% cases are peracute and present as '''sudden death'''. This may be due to sequelae such as adrenal gland involvement or rupture of abomasal ulcers etc. |
| − | Other signs are multisystemic and non-specific: | + | Other signs are '''multisystemic''' and non-specific: |
| − | Cardiovascular – | + | Cardiovascular – dysrhythmmias, jugular distension, tachycardia, murmurs |
| − | Gastrointestinal – | + | Gastrointestinal – anorexia, hepatomegaly, dysphagia, constipation or diarrhoea, abomasal tympany, hypomotility |
Retrobulbar tumours sometimes cause blindness and other ocular signs. | Retrobulbar tumours sometimes cause blindness and other ocular signs. | ||
| Line 50: | Line 45: | ||
Neurological signs and lameness also sometimes occur due to local tumour growth. | Neurological signs and lameness also sometimes occur due to local tumour growth. | ||
| − | Animals are usually afebrile. | + | Animals are usually '''afebrile'''. |
Infected animals become permanent carriers. | Infected animals become permanent carriers. | ||
| Line 57: | Line 52: | ||
Persistent lymphocytosis is defined by an increase in total lymphocyte count by 3 times the normal standard deviation above normal, persisting for at least three months with no clinical signs of neoplastic lesions. This is thought to represent 1/3 of all BLV infected cattle. | Persistent lymphocytosis is defined by an increase in total lymphocyte count by 3 times the normal standard deviation above normal, persisting for at least three months with no clinical signs of neoplastic lesions. This is thought to represent 1/3 of all BLV infected cattle. | ||
| − | =Diagnosis= | + | ==Diagnosis== |
| + | Identification of enlarged lymph nodes without a raised rectal temperature may instigate suspicion. | ||
| + | |||
| + | A range of serological tests are available although in calves none are able to distinguish between maternal and endogenous antibodies. Antibodies may not be present for up to 12 weeks following onset of infection. In the UK, seropositive calves were found to be due to the use of an imported colostrum replacer from an EBL endemic country <ref>DEFRA, 2010. http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ebl/colostrum.htm. Accessed 08/07/2012</ref>. | ||
| + | |||
| + | '''Agar Gel Immuno Diffusion (AGID)''' is the official standard test approved by most governments. | ||
| − | + | Radioimmunoassay and serum or bulk milk [[ELISA testing|ELISA]] are also available. | |
| − | + | PCR can also be performed on peripheral blood lymphocytes. This test may be required to distinguish seropositive calves as either infected or having maternally derived antibodies. | |
| − | =References= | + | Multiple, firm white tumours may be present in any organ on post-mortem examination. In young animals, the common sites are the kidneys, thymus, liver, spleen, and lymph nodes. In adults, the heart, abomasum, and spinal cord are often involved. |
| + | |||
| + | ==Treatment== | ||
| + | None Available | ||
| + | |||
| + | ==Control== | ||
| + | Testing and culling using the AGID test and PCR for young calves is effective, but not economically viable in high prevalence herds. In these circumstances, prevention of transmission between animals by direct contact with blood is the key focus. Some countries and states have mandatory management and monitoring practices in place which must be obeyed. | ||
| + | |||
| + | Feeding young calves milk from seronegative dams can prevent further transmission of BLV, although in high prevalence herds, frozen colostrum from infected dams has been used in order to provide passive immunity to the virus, while reducing lymphocyte infectivity. Note that these calves will then have a positive AGID result and therefore may be refused for export. | ||
| + | |||
| + | No vaccine is currently available for BVL/EBL. | ||
| + | |||
| + | As the role of insect vectors is poorly understood, control of these is prudent as part of a control plan. | ||
| + | |||
| + | |||
| + | {{Learning | ||
| + | |flashcards = [[Bovine Leukaemia Virus Flashcards]] | ||
| + | }} | ||
| + | |||
| + | |||
| + | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | Merck Veterinary Manual, Bovine Leukosis, accessed online 02\06\2011 @ http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/54800.htm&word=leukosis | ||
| + | |||
| + | {{CABI source | ||
| + | |datasheet = [http://www.cabi.org/ahpc/?compid=3&dsid=91731&loadmodule=datasheet&page=2144&site=160 bovine leukemia virus] and [http://www.cabi.org/ahpc/Default.aspx?site=160&page=2144&LoadModule=datasheet&CompID=3&dsID=91714 enzootic bovine leukosis] | ||
| + | |date =30 May 2011 | ||
| + | }} | ||
| + | <br><br><br> | ||
| + | |||
| + | {{Nick Lyons | ||
| + | |date = July 8, 2012}} | ||
| + | |||
| + | [[Category:Retroviridae]] | ||
| + | [[Category:Lymphoreticular and Haematopoietic Diseases - Cattle]] | ||
| + | [[Category: CABI Expert Review]] | ||
| + | [[Category:Nick Lyons reviewed]] | ||
Latest revision as of 11:45, 10 July 2012
Also Known As: Enzootic Bovine Leucosis — Bovine Leukosis — EBL — BLV — BoLV — Bovine Leukemia — Lymphosarcoma — Sporadic Bovine Leukosis — Bovine Malignant Lymphoma
Introduction
Bovine leukaemia virus is a retrovirus causing two specific diseases: Bovine lymphosarcoma and Persistent lymphocytosis.
Bovine lymphosarcoma is fatal while persistent lymphocytosis is not usually so. However, those affected with lymphosarcoma may or may not have been through the persistent lymphocytosis stage [1].
Bovine leukosis is not transmissible to humans.
This disease is notifiable to the World Organisation for Animal Health (OIE)
Signalment
Cattle are thought to be the only species naturally susceptible, and prevalence rates are higher in dairy breeds than beef. The majority of infected animals are more than 2 years of age, with younger animals developing sporadic bovine leukosis which is thought to be unrelated.
Sheep are susceptible to experimental infection which appears more pathogenic in this species.
Manifestation of the fatal neoplastic lymphosarcoma form of disease also appears better represented in dairy cattle.
Distribution
BLV is globally distributed, but prevalence widely varies. The UK is currently free of infection evidenced by government funded bulk milk antibody surveillance and investigations of tumours found in live or dead animals [2].
While 89% dairy operations in the USA showed seropositive results and 43.5% of all USA dairy cattle were seropositive [3], seroprevalence in the EU rarely exceeds 0.5-1.5%. [4]
Transmission
Transmission of bovine leukaemia virus is mainly horizontal. Iatrogenic procedures are an important route as they facilitate transfer of contaminated blood between individuals, e.g. vaccinations, dehorning, rectal examinations and surgical procedures. Natural transmission by direct contact requires contact with infected blood, exudates or tissues which enter through broken skin. Vertical transmission is most likely from colostrum/milk and is less important, although it does provide a method for control.
Clinical Signs
Lymphosarcoma
The most common presentation is that of enlarged lymph nodes which may cause multiple palpable swellings in the pharynx, flanks and limbs. This is often an early indicator of disease.
5-20% cases are peracute and present as sudden death. This may be due to sequelae such as adrenal gland involvement or rupture of abomasal ulcers etc.
Other signs are multisystemic and non-specific:
Cardiovascular – dysrhythmmias, jugular distension, tachycardia, murmurs
Gastrointestinal – anorexia, hepatomegaly, dysphagia, constipation or diarrhoea, abomasal tympany, hypomotility
Retrobulbar tumours sometimes cause blindness and other ocular signs.
Neurological signs and lameness also sometimes occur due to local tumour growth.
Animals are usually afebrile.
Infected animals become permanent carriers.
Persistent Lymphocytosis
Persistent lymphocytosis is defined by an increase in total lymphocyte count by 3 times the normal standard deviation above normal, persisting for at least three months with no clinical signs of neoplastic lesions. This is thought to represent 1/3 of all BLV infected cattle.
Diagnosis
Identification of enlarged lymph nodes without a raised rectal temperature may instigate suspicion.
A range of serological tests are available although in calves none are able to distinguish between maternal and endogenous antibodies. Antibodies may not be present for up to 12 weeks following onset of infection. In the UK, seropositive calves were found to be due to the use of an imported colostrum replacer from an EBL endemic country [5].
Agar Gel Immuno Diffusion (AGID) is the official standard test approved by most governments.
Radioimmunoassay and serum or bulk milk ELISA are also available.
PCR can also be performed on peripheral blood lymphocytes. This test may be required to distinguish seropositive calves as either infected or having maternally derived antibodies.
Multiple, firm white tumours may be present in any organ on post-mortem examination. In young animals, the common sites are the kidneys, thymus, liver, spleen, and lymph nodes. In adults, the heart, abomasum, and spinal cord are often involved.
Treatment
None Available
Control
Testing and culling using the AGID test and PCR for young calves is effective, but not economically viable in high prevalence herds. In these circumstances, prevention of transmission between animals by direct contact with blood is the key focus. Some countries and states have mandatory management and monitoring practices in place which must be obeyed.
Feeding young calves milk from seronegative dams can prevent further transmission of BLV, although in high prevalence herds, frozen colostrum from infected dams has been used in order to provide passive immunity to the virus, while reducing lymphocyte infectivity. Note that these calves will then have a positive AGID result and therefore may be refused for export.
No vaccine is currently available for BVL/EBL.
As the role of insect vectors is poorly understood, control of these is prudent as part of a control plan.
| Bovine Leukaemia Virus Learning Resources | |
|---|---|
 Test your knowledge using flashcard type questions |
Bovine Leukaemia Virus Flashcards |
References
- ↑ Radostitis et al (2007). Veterinary Medicine 10th Edition, Sauders Elsevier, Chapter 21
- ↑ DEFRA (2010). http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ebl/index.htm. Accessed 08/07/2012
- ↑ USDA/APHIS/Veterinary Services, (1997). High prevalence of BLV in US dairy herds. Fort Collins, CO, USA: USDA: APHIS:VS Centers For Epidemiology and Animal Health.
- ↑ Lorenz, R. J., Straub, O. C. (1987) The epidemiology of enzootic bovine leukosis. In: Burny A, Mammerick, M, eds. Enzootic bovine leukosis and bovine leukemia virus. Boston, USA: Martinus Nijhoff Publishing, 51-68
- ↑ DEFRA, 2010. http://archive.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/ebl/colostrum.htm. Accessed 08/07/2012
Merck Veterinary Manual, Bovine Leukosis, accessed online 02\06\2011 @ http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/54800.htm&word=leukosis

|
This article was originally sourced from The Animal Health & Production Compendium (AHPC) published online by CABI during the OVAL Project. The datasheet was accessed on 30 May 2011. |
| This article has been expert reviewed by Nick Lyons MA VetMB CertCHP MRCVS Date reviewed: July 8, 2012 |