Difference between revisions of "Tapeworm - Donkey"
(No difference)
| |
Latest revision as of 09:25, 21 October 2016


Introduction
Tapeworms or cestodes belong to the group of parasites called flat worms or platyhelminths. Anoplocephala perfoliata, A. magna and Anaplacepholoides mammilana are the common species affecting equids. These flatworms are typically about 1.5cm wide and range in size from 8-25cm long with A. magna being the largest and A. mammilana the smallest. Recent studies have shown that A. perfoliata is the most common equine tapeworm (Neilsen, 2016).
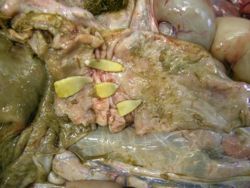
The tapeworm body is composed of a scolex (head) and a flattened strobila (body). The scolex has four suckers which form an attachment to the intestinal wall of the host. Tapeworms have no mouth parts or unified digestive tract and instead absorb nutrients through the proglottid (body segment) walls.
A. perforliata’s site of predilection is usually around the ileo-caecal junction whereas A. magna and A. mammilana favour attachment in the small intestine, and in the case of A. mammilana occasionally the stomach. A. perfoliata is prevalent worldwide and can be found in any demographic group. It was once thought to be of low clinical significance but it is now understood that an infection can be implicated in the development of severe gastrointestinal disruptions including intussusception (Neilsen, 2016).
Lifecycle
The tapeworm has a relatively long lifecycle, involving an intermediate host (oribatid mites) and taking approximately 2-3 (but maybe as long as 6 months) to complete. Grazing animals ingest oribatid mites infected with cysticercoid (larval stage) which are released once inside the digestive tract. The cysticercoid develops into a mature adult worm inside the host. The strobila of the mature worm is composed of proglottids in which eggs are formed, the gravid proglottids separate from the main body and are excreted out in the faeces. Eggs are eaten by free living oribatid mites within which they develop into cysticercoids and the lifecycle continues.
Diagnosis
Clinical signs
Tapeworms have been incriminated as a cause of intestinal intussusceptions, caecal perforation leading to peritonitis, intestinal obstruction and colic. The severity and depth of ulcerative lesions of the mucosa increase as the number of tapeworms attached in the area increases (Williamson et al, 1997: Neilsen, 2016). Although the pathogenic effect of tapeworm infection in donkeys has not been studied experimentally, similar lesions, particularly ulcerations of the ileocaecal junction, have been observed in donkeys.
At The Donkey Sanctuary 3% of donkeys admitted have evidence of tapeworm infection on faecal examination. Post-mortem evidence suggests an even lower incidence. The low incidence may be due to the poor habitat for oribatid mites on its farms. On the other hand, a study made in Ethiopia has shown a high prevalence of anoplocephalosis in donkeys, particularly in highland areas where permanent pasture management is often practised and oribatid mites are abundant(Getachew et al, 2012).
Clinical signs are well documented in horses and range from weight loss, lack of energy and anaemia, to severe GI disturbances in cases of heavy infection. Pathological reactions characterised by hyperaemia, mucosal thickening and necrotic ulcers around the attachment sites have been reported (Neilsen, 2016). Clinical signs in donkeys are less well understood, as the incidence rate appears to be lower and few clinical cases are seen.
Laboratory tests
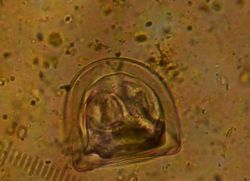
Diagnosis using conventional faecal floatation methods has low sensitivity, due to uneven egg distribution in faeces. However, it is highly specific, as the tapeworm eggs are distinctively angular and readily identified.
Serological diagnosis with enzyme-linked immunosorbent assay (ELISA) using excretory/secretory (E/S) antigens has been developed to detect the antibody in serum (Proudman and Trees, 1996) and in saliva (Lightbody et al, 2016) although the accuracy of its application in donkeys is uncertain. The Donkey Sanctuary does not use these diagnostic techniques.
Treatment
Historically many equine owners have administered an anthelmintic once or twice a year (typically early spring and/or late autumn), particularly if there have been cases on site in previous years. At The Donkey Sanctuary, however, treatment for tapeworm is only carried out when there is positive identification of eggs in the faeces. Treatment options include:
- Pyrantel at twice the dose rate for roundworms.
- Praziquantel is also effective against tapeworm in donkeys. Recent research (Getachew et al 2013) has found praziquantel to have an efficacy rate of over 99% for up to 16 weeks post treatment when given at an oral dose rate of 1mg/kg.
The Donkey Sanctuary does not advocate the use of combination de-wormers which combine a macrocyclic lactone (moxidectin or ivermectin) with praziquantel. These preparations are not licensed for use in donkeys and anecdotal evidence suggests they may be poorly tolerated.
Control
Areas of permanent pasture land are thought to have a higher prevalence rate so pasture rotation would be beneficial in preventing/lowering cestode infection. As always good pasture management and hygiene is beneficial. Owners and vets caring for animals on sites that have housed animals harbouring a cestode infection in the past may consider annual or biannual treatment as a preventative option, but this would not be recommended as the first course of action with no previous history to cause concern.
Literature Search
Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation).
Tapeworm in donkeys publications
References
- Trawford, A. and Getachew, M. (2008) Parasites In Svendsen, E.D., Duncan, J. and Hadrill, D. (2008) The Professional Handbook of the Donkey, 4th edition, Whittet Books, Chapter 6
- Getachew, M.A., Innocent, G., Proudman, C.J., Trawford, A., Feseha, G., Reid, S.J.W., Faith, B. and Love, S. (2012). Equine cestodosis: a sero-epidemiological study of Anoplocephala perfoliata infection in Ethiopia. Vet Res Commun, 36:98-98.
- Getachew, M., Innocent, G., Trawford, A., Feseha, G., Reid, S.J.W., and Love, S. (2006). ‘Equine cestodosis: a sero-epidemiological study of Anoplocephala perfoliata infection in Ethiopia’. Proceedings of the 9th Congress of World Equine Veterinary Association. 22-26 January 2006. Marrakech, Morocco.
- Getachew, M., Innocent, G., Proudman, C., Trawford, A., Gebreab, F., Reid, S.,Burden, F., Love, S. September 2013. Field efficacy of praziquantel oral paste against naturally acquired equine cestodes in Ethiopia. Parasitology Research, 112: 141-146.
- Proudman, C.J., Trees, A.J. (1996). ‘Use of excretory/secretory antigens for the serodiagnosis of A. perfoliata cestodosis’. Vet. Parasitol. 61: 239-247.
- Williamson, R.M.C., Gasser, R.B., Middleton, D., and Beveridge, I. (1997). ‘The distribution of Anoplocephala perfoliata in the intestine of the horse and associated pathological changes’. Vet.Parasitol. 73: 225-241
- Nielsen, M.K., 2016. Equine tapeworm infections: Disease, diagnosis and control. Equine Vet Education, 28(7):388-395.
- Lightbody, K.L., Davis, P.J., Austin, C.J., 2016. Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses. Vet Clin Pathol 45 (2): 335–346.
|
|
This section was sponsored and content provided by THE DONKEY SANCTUARY |
|---|
