Difference between revisions of "Small Animal Emergency and Critical Care Medicine: Self-Assessment Color Review, Second Edition, Q&A 04"
Jump to navigation
Jump to search
(No difference)
| |
Latest revision as of 09:34, 26 November 2018
| This question was provided by CRC Press. See more case-based flashcards |

|
Student tip: This case is ideal for applying the theory of acid/base balance to a real life situation. |
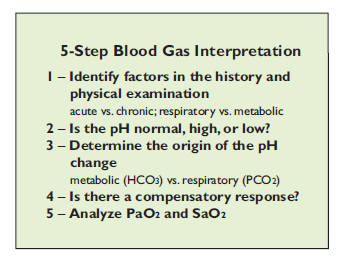
Assessing venous blood gas provides information regarding the acid–base balance of the critical patient. An arterial blood gas allows evaluation of the efforts of ventilation (PaCO2) and oxygenation (PaO2). There are 5 steps to use when interpreting the results from blood gas analysis (see table above).
| Question | Answer | Article | |
| List at least four physiologic effects of acidosis. | Hyperventilation, shift of oxyhemoglobin curve to right, decreased 2,3 DPG levels in RBCs, sympathetic overactivity, resistance to action of catecholamines, peripheral arterial vasodilation, peripheral venoconstriction, pulmonary vasoconstriction, depressed myocardial contractility, cerebral vasodilation (high PaCO2), shift of K+ out of cells causing hyperkalemia, increased extracellular phosphate concentration.
|
Link to Article | |
| List at least four physiologic effects of alkalosis. | Shift of oxyhemoglobin dissociation curve to left, inhibition of respiratory drive, cerebral vasoconstriction, increased neuromuscular excitability, hypokalemia.
|
Link to Article | |
| Which component of the blood gas is responsible for reflecting respiratory acidosis/alkalosis? As this value increases, do H+ ions increase or decrease? What action of the body affects this component? | PaCO2. A drop or rise in PaCO2 results in a drop or rise of H+ ion concentration respectively. PCO2 is affected by alveolar ventilation.
|
Link to Article | |
| Which component of the blood gas is responsible for reflecting metabolic acidosis/alkalosis? As this value increases, do H+ ions increase or decrease? What action of the body affects this component? | HCO3. The HCO3 is inversely related to the H+ ion concentration (e.g. drop in HCO3 leads to increase in H+ and rise in HCO3 results in drop in H+). HCO3 is affected by retention or excretion by the kidneys.
|
Link to Article | |
| Administration of sodium bicarbonate may be required in severe metabolic acidosis. What are the pros and cons of sodium bicarbonate? | Pros: rapid treatment of metabolic acidosis and its deleterious consequences. Cons: increased mortality reported; shift in ionized calcium and cellular potassium; paradoxical intracellular acidosis; hypernatremia; rebound alkalemia.
|
Link to Article | |
| What therapy should be initiated in severe respiratory acidosis and in severe respiratory alkalosis? | Increase alveolar ventilation; rebreathe exhaled air (breathe into a bag).
|
Link to Article | |
To purchase the full text with your 20% off discount, go to the CRC Press Veterinary website and use code VET18.