Difference between revisions of "Lizard Blood Collection"
(Undo revision 115459 by Yensen Hartanto (talk)) |
|||
| (15 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{unfinished}} |
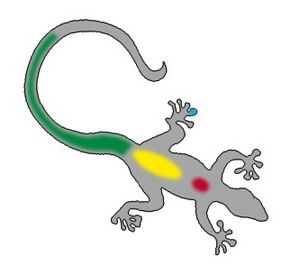
| − | [[Image:Lizard_bloodsites2.jpg| | + | [[Image:Lizard_bloodsites2.jpg|300px|thumb|right|'''Blood collection sites in a lizard''' (Copyright © RVC and its licensors, Sean Bobbit, Sue Evans, Andrew Devare and Claire Moore. All rights reserved)]] |
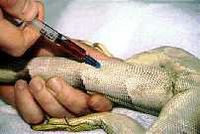
| − | [[Image:Bloodtake_1.jpg|200px|thumb|right|'''Blood taking from the ventral tail vein''' (Copyright © RVC)]] | + | [[Image:Bloodtake_1.jpg|200px|thumb|right|'''Blood taking from the ventral tail vein''' (Copyright © RVC and its licensors, Sean Bobbit, Sue Evans, Andrew Devare and Claire Moore. All rights reserved)]] |
| − | The ventral tail vein is the preferred site of venipuncture in lizards but be very careful in species that are capable of [[ | + | The ventral tail vein is the preferred site of venipuncture in lizards but be very careful in species that are capable of [[Lizard Musculoskeletal System|autotomy]]. Analysis of blood include [[Lizard and Snake Haemotology|haematology]] and biochemistry. |
==Sites== | ==Sites== | ||
| Line 10: | Line 10: | ||
===Ventral Tail Vein=== | ===Ventral Tail Vein=== | ||
| − | Ensure that the lizard is adequately restrained. When | + | Ensure that the lizard is adequately restrained. When anaesthetised position in dorsal recumbency. |
Unsedated iguanas can be placed on an examination table with the tail held over the edge and approached from below or hung from a wire cage door. | Unsedated iguanas can be placed on an examination table with the tail held over the edge and approached from below or hung from a wire cage door. | ||
| Line 19: | Line 19: | ||
*Direct needle at 45 to 90 degrees at ventral midline. | *Direct needle at 45 to 90 degrees at ventral midline. | ||
*Advance the needle while maintaining slight negative pressure until blood is observed or contact is made with the ventral surface of the vertebrae. | *Advance the needle while maintaining slight negative pressure until blood is observed or contact is made with the ventral surface of the vertebrae. | ||
| − | *If not successful, withdraw slightly or redirect the needle so that it is definitely in the midline. | + | *If not successful, withdraw slightly or redirect the needle so that it is definitely in the midline. |
===Ventral Abdominal Vein=== | ===Ventral Abdominal Vein=== | ||
| − | The | + | The ventral abdominal vein is a large vein 1-2 mm within the coelomic cavity on ventral midline between the umbilical scar and pelvic inlet. Care is advised since the coelomic cavity has a large capacity if there is continued bleeding after venipuncture. |
===Toenail Clip=== | ===Toenail Clip=== | ||
| Line 34: | Line 34: | ||
==Amounts== | ==Amounts== | ||
| − | In reptiles the total blood volume varies with species but is approximately 5-8% bodyweight. The maximum that can be drawn safely is 10% of the total blood volume. A 100 g reptile can therefore have 0.5 ml safely taken. (Weigh accurately and make the calculations before blood is withdrawn!). Though microtechniques for biochemistry are available in some laboratories, it is generally advisable to take 1.5 ml of blood for complete | + | In reptiles the total blood volume varies with species but is approximately 5-8% bodyweight. The maximum that can be drawn safely is 10% of the total blood volume. A 100 g reptile can therefore have 0.5 ml safely taken. (Weigh accurately and make the calculations before blood is withdrawn!). Though microtechniques for biochemistry are available in some laboratories, it is generally advisable to take 1.5 ml of blood for complete haematology and biochemistry. |
==Blood handling== | ==Blood handling== | ||
| − | Requirements for blood handling: | + | Requirements for blood handling: two blood collection tubes with lithium heparin (a 0.5 ml orange top tube and a 1.0ml green top tube with gel) and four microscope coverslips (or three microscope slides). Put 0.5 ml of blood into the 0.5 ml tube (for haematology) and 1.0 ml into the 1.0 ml tube (for biochemistry). From the small amount of blood left in the needle make two blood films. Air-dried thin smears give superior cell morphology compared to samples with anticoagulants; EDTA may lyse cells and heparin gives a bluish tinge. Slide smears are adequate but coverslip smears are superior. Centrifuge the 1.0 ml tube and harvest the plasma which is above the gel while the cells are below it. |
| − | + | [[Category:Lizard Specimen Collection|B]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Category:Lizard | ||
Revision as of 18:05, 17 March 2010
| This article is still under construction. |
The ventral tail vein is the preferred site of venipuncture in lizards but be very careful in species that are capable of autotomy. Analysis of blood include haematology and biochemistry.
Sites
Ventral Tail Vein
Ensure that the lizard is adequately restrained. When anaesthetised position in dorsal recumbency.
Unsedated iguanas can be placed on an examination table with the tail held over the edge and approached from below or hung from a wire cage door.
Follow these steps:
- Prepare skin aseptically.
- Use needle size - 21 to 25 gauge.
- Direct needle at 45 to 90 degrees at ventral midline.
- Advance the needle while maintaining slight negative pressure until blood is observed or contact is made with the ventral surface of the vertebrae.
- If not successful, withdraw slightly or redirect the needle so that it is definitely in the midline.
Ventral Abdominal Vein
The ventral abdominal vein is a large vein 1-2 mm within the coelomic cavity on ventral midline between the umbilical scar and pelvic inlet. Care is advised since the coelomic cavity has a large capacity if there is continued bleeding after venipuncture.
Toenail Clip
A toenail clip may be the only method of blood collection in smaller lizards. Collect into a capillary tube.
Heart
The heart is not recommended for cardiocentesis because of the risk of haemopericardium.
Amounts
In reptiles the total blood volume varies with species but is approximately 5-8% bodyweight. The maximum that can be drawn safely is 10% of the total blood volume. A 100 g reptile can therefore have 0.5 ml safely taken. (Weigh accurately and make the calculations before blood is withdrawn!). Though microtechniques for biochemistry are available in some laboratories, it is generally advisable to take 1.5 ml of blood for complete haematology and biochemistry.
Blood handling
Requirements for blood handling: two blood collection tubes with lithium heparin (a 0.5 ml orange top tube and a 1.0ml green top tube with gel) and four microscope coverslips (or three microscope slides). Put 0.5 ml of blood into the 0.5 ml tube (for haematology) and 1.0 ml into the 1.0 ml tube (for biochemistry). From the small amount of blood left in the needle make two blood films. Air-dried thin smears give superior cell morphology compared to samples with anticoagulants; EDTA may lyse cells and heparin gives a bluish tinge. Slide smears are adequate but coverslip smears are superior. Centrifuge the 1.0 ml tube and harvest the plasma which is above the gel while the cells are below it.