Difference between revisions of "Erythropoiesis"
Fiorecastro (talk | contribs) |
|||
| (11 intermediate revisions by 6 users not shown) | |||
| Line 3: | Line 3: | ||
[[Erythrocytes|Erythrocytes]] contain no nucleus and are thus only produced from stem cells. During the fetal stage production is in both the [[Liver - Anatomy & Physiology|liver]] and [[Spleen - Anatomy & Physiology|spleen]] however production is transferred to the [[Bone Marrow - Anatomy & Physiology|bone marrow]] ([[Bone Marrow - Anatomy & Physiology#Red marrow|red marrow]]) in the final stages of gestation. Initially erythropoiesis occurs in all bones, however after puberty production is limited to membranous bones (ribs, vertebrae, pelvic bones etc.) as the long bones contain adipose tissue in place of red marrow. | [[Erythrocytes|Erythrocytes]] contain no nucleus and are thus only produced from stem cells. During the fetal stage production is in both the [[Liver - Anatomy & Physiology|liver]] and [[Spleen - Anatomy & Physiology|spleen]] however production is transferred to the [[Bone Marrow - Anatomy & Physiology|bone marrow]] ([[Bone Marrow - Anatomy & Physiology#Red marrow|red marrow]]) in the final stages of gestation. Initially erythropoiesis occurs in all bones, however after puberty production is limited to membranous bones (ribs, vertebrae, pelvic bones etc.) as the long bones contain adipose tissue in place of red marrow. | ||
| − | Erythrocyte stem cells contain no haemoglobin and it is only after several cell divisions that pro-erythrocyte haemoglobin starts to be generated within the cells. When the haemoglobin levels are at the correct concentration the nucleus reduces in size and is removed from cell. Cells at this stage still have ribosomes and other organelles and stain differently to mature erythrocytes; they are known as | + | Erythrocyte stem cells contain no haemoglobin and it is only after several cell divisions that pro-erythrocyte haemoglobin starts to be generated within the cells. When the haemoglobin levels are at the correct concentration the nucleus reduces in size and is removed from cell. Cells at this stage still have ribosomes and other organelles and stain differently to mature erythrocytes; they are known as reticulocytes. Reticulocytes contain some RNA and continue to produce haemoglobin. After a few days these mature having reached a final haemoglobin concentration of 34%. |
| − | Reticulocytes and mature [[Erythrocytes|erythrocytes]] leave the [[Bone Marrow - Anatomy & Physiology|bone marrow]] by ‘squeezing’ through the capillary endothelial cells. Precursors to these stages cannot change shape and therefore remain | + | Reticulocytes and mature [[Erythrocytes|erythrocytes]] leave the [[Bone Marrow - Anatomy & Physiology|bone marrow]] by ‘squeezing’ through the capillary endothelial cells. Precursors to these stages cannot change shape and therefore remain confoned to the bone marrow. |
==Development== | ==Development== | ||
| Line 66: | Line 66: | ||
Production of [[Erythrocytes|erythrocytes]] is regulated by '''erythropoietin''' (EPO) which is produced in the yolk sac, [[Liver - Anatomy & Physiology|liver]] and kidney from embryonic life until early neonatal life. In the adult it is produced only in the kidneys. Erythropoietin is a glycoprotein hormone and is controled by a negative feedback mechanism. Normal levels are low with sufficient amounts to maintain a basal level of new erythrocyte production. If blood oxygen concentration falls, the release of erythropoietin rises. | Production of [[Erythrocytes|erythrocytes]] is regulated by '''erythropoietin''' (EPO) which is produced in the yolk sac, [[Liver - Anatomy & Physiology|liver]] and kidney from embryonic life until early neonatal life. In the adult it is produced only in the kidneys. Erythropoietin is a glycoprotein hormone and is controled by a negative feedback mechanism. Normal levels are low with sufficient amounts to maintain a basal level of new erythrocyte production. If blood oxygen concentration falls, the release of erythropoietin rises. | ||
| − | EPO is transported from kidneys to [[Bone Marrow - Anatomy & Physiology|bone marrow]] where it acts upon receptors on the CFU-E’s and causes differentiation into erythrocyte precursors. It also increases the rate of division and maturation of developing erythrocyte precursors by increasing | + | EPO is transported from kidneys to [[Bone Marrow - Anatomy & Physiology|bone marrow]] where it acts upon receptors on the CFU-E’s and causes differentiation into erythrocyte precursors. It also increases the rate of division and maturation of the developing erythrocyte precursors by increasing gene transcription. Thus it is not the number of [[Erythrocytes|erythrocytes]] but the oxygen concentration that regulates its release. EPO release can be affected by any form of renal pathology. |
| − | + | ===Factors involved=== | |
| + | Erythropoietin production is directly and indirectly (via regulatory genes) increased by HIF-1 (Hypoxia inducible factor 1) which is a transcription activator that is oxygen sensitive. | ||
| + | Inflammatory induced release of interleukins reduces the secretion of erythropoietin. | ||
[[Category:Haematopoiesis]] | [[Category:Haematopoiesis]] | ||
Revision as of 13:08, 28 September 2010
Introduction
Erythrocytes contain no nucleus and are thus only produced from stem cells. During the fetal stage production is in both the liver and spleen however production is transferred to the bone marrow (red marrow) in the final stages of gestation. Initially erythropoiesis occurs in all bones, however after puberty production is limited to membranous bones (ribs, vertebrae, pelvic bones etc.) as the long bones contain adipose tissue in place of red marrow.
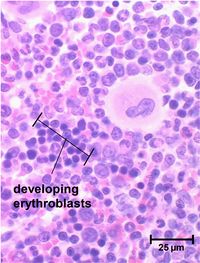
Erythrocyte stem cells contain no haemoglobin and it is only after several cell divisions that pro-erythrocyte haemoglobin starts to be generated within the cells. When the haemoglobin levels are at the correct concentration the nucleus reduces in size and is removed from cell. Cells at this stage still have ribosomes and other organelles and stain differently to mature erythrocytes; they are known as reticulocytes. Reticulocytes contain some RNA and continue to produce haemoglobin. After a few days these mature having reached a final haemoglobin concentration of 34%.
Reticulocytes and mature erythrocytes leave the bone marrow by ‘squeezing’ through the capillary endothelial cells. Precursors to these stages cannot change shape and therefore remain confoned to the bone marrow.
Development
Erythrocyte development is divided into several stages and at each stage the cell will divide several times. As bone marrow does not act as storage, all erythrocytes produced are immediately released into the circulation.
Stem cell
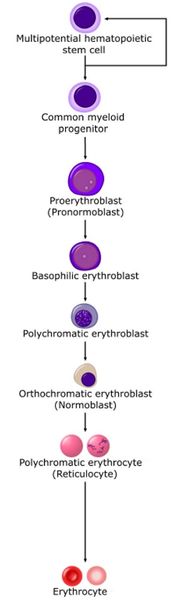
Stem cells give rise to precursors which eventually mature to form the fully developed erythrocyte. Initially, the multipotential myeloid stem cell (CFU-GEMM) differentiates into the erythroid CFU (CFU-E). This develops into the first erythrocyte precursor, the proerythroblast. The development process is controlled and influenced by a number of different factors including erythropoietin, IL-3, IL-4 and granulocyte-macrophage colony stimulating factor (GM-CSF).
Nomenclature
The naming of the stages of the erythrocyte precursors varies.
The main headings for this section refer to each stage in terms of a histological description, namely the proerythroblast, basophilic erythroblast, polychromatic erythroblast, orthochromatic erythroblast and polychromatophilic erythrocyte.
Another classification of the stages is based upon the CFU-E being referred to as ‘rubri’. This system gives all developing blood cell lineages similar names. For example the first stage for erythrocytes is referred to as a [rubri]blast and the first stage for lymphocytes is called a [lympho]blast. The stages for the erythrocyte are rubriblast, prorubriblast, rubricyte and metarubricye.
Finally the stages can also be named according to the development of the normoblast stage. This gives the stages pronormoblast, early normoblast, intermediate normoblast, late normoblast, polychromatic cell.
Stages of Development
Proerythroblast
The first erythrocyte precursor produced directly from the CFU-GEMM under the influence of erythropoietin. It has a large nucleus with free ribosomes in the cytoplasm giving the cytoplasm a basophilic appearance.
Alternative nomenclature: Pronormoblast or Rubriblast
Basophilic erythroblast
Smaller than the proerythroblast with a smaller nucleus but a more basophilic cytoplasm due to increased numbers of ribosomes in the cytoplasm. These ribosomes are involved in the production of haemoglobin.
Alternative nomenclature: Early normoblast or Rubriblast
Polychromatic erythroblast
This is the last precursor cell capable of mitosis and is smaller than the basophilic erythroblast. Its cytoplasm appears greyer due to the increased acidophilic staining caused by the presence of haemoglobin.
Alternative nomenclature: Intermediate normoblast or Prorubricyte
Orthochromatic erythroblast
Also called a normoblast. It is incapable of cell division and is only slightly larger than a mature erythrocyte but it does contain a small dense nucleus.
Alternative nomenclature: Late normoblast or Rubricyte
Polychromatophilic erythrocyte
Also called a reticulocyte and is formed when the nucleus is extruded from the normoblast. It still contains some ribosomes and therefore retains a slight basophilic stain. The clustering of the ribosomes forms a reticular network giving the name, reticulocyte. These cells can carry oxygen and enter the blood stream and are found in low concentrations in normal blood.
Alternative nomenclature: Polychromatic cell or Metarubricyte
Erythrocyte
Commonly called the red blood cell. It is the final product of erythropoiesis and is released from the bone marrow into circulation.
Alternative nomenclature: None
Nutritional factors
Normal erythrocyte production requires protein, copper, iron, folic acid and vitamins B6 and B12. Deficiencies lead to inadequate production of haemoglobin.
Iron
Iron is found within each haem component of haemoglobin and binds oxygen.
Folic acid and vitamin B12
These elements are required for DNA synthesis and are important for the formation of new erythrocytes. Deficiencies leads to the formation of macrocytes, which are enlarged erythrocytes with a higher than normal mean corpuscular volume (MCV).
Regulation
Production of erythrocytes is regulated by erythropoietin (EPO) which is produced in the yolk sac, liver and kidney from embryonic life until early neonatal life. In the adult it is produced only in the kidneys. Erythropoietin is a glycoprotein hormone and is controled by a negative feedback mechanism. Normal levels are low with sufficient amounts to maintain a basal level of new erythrocyte production. If blood oxygen concentration falls, the release of erythropoietin rises.
EPO is transported from kidneys to bone marrow where it acts upon receptors on the CFU-E’s and causes differentiation into erythrocyte precursors. It also increases the rate of division and maturation of the developing erythrocyte precursors by increasing gene transcription. Thus it is not the number of erythrocytes but the oxygen concentration that regulates its release. EPO release can be affected by any form of renal pathology.
Factors involved
Erythropoietin production is directly and indirectly (via regulatory genes) increased by HIF-1 (Hypoxia inducible factor 1) which is a transcription activator that is oxygen sensitive. Inflammatory induced release of interleukins reduces the secretion of erythropoietin.