Difference between revisions of "Equine Togaviral Encephalitis"
| Line 14: | Line 14: | ||
====Aetiology==== | ====Aetiology==== | ||
| − | [[Equine Encephalitis Virus| | + | [[Equine Encephalitis Virus|See this page for details of the causal pathogens]]. The same virus strains can infect poultry and other farmed birds including quail and ratites. Some strains are potential agents of biowarfare or bioterrorism (Steele and Twenhafel, 2010). |
====Epidemiology==== | ====Epidemiology==== | ||
Revision as of 17:30, 7 July 2010
| This article is still under construction. |
Description
NOTIFIABLE and ZOONOTIC infectious mosquito-borne disease of equidae affecting the central nervous system (CNS).
Aetiology
See this page for details of the causal pathogens. The same virus strains can infect poultry and other farmed birds including quail and ratites. Some strains are potential agents of biowarfare or bioterrorism (Steele and Twenhafel, 2010).
Epidemiology
Disease associated with EEE, WEE and VEE is largely restricted to the Western Hemisphere, ranging from temperate to desert climates. EEE in the United States is mainly seen in the Southeastern United States but has been detected in all states east of the Mississippi River and some Western states. Large outbreaks of WEE have been described in California and other Western states but the incidence of clinical disease in these areas has experienced a dramatic decrease. The reason for this unknown but may be due to geographical variation in virulence. Equine disease associated with WEE is rare on the Eastern seaboard ofthe United States. VEE virus is a very important human andveterinary pathogen in the Western Hemipshoere that can cause large outbreaks of disease in humans and horses over large geopgrahic areas. VEE has spread into Central America, causing devastating epidemics as far north as Texas. The disease distribution is determined by climatic conditions as well as agricultural practices, such as irrigation, which favour the life cycle and spread of mosquitoes. Transfer is via vector: mostly through mosquito salivary transfer. WEE and VEE may also be transmitted via nasal secretions but this is less likely. Disease amplification occurs during the viraemic phase which lasts until nervous signs develop. Amplification from horses is unlikely with EEE and WEE but occurs with VEE in association with a relatively high viraemia. Ocular and nasal discharges from infected horses cotnain high concentrations of VEE. Zoonotic spread has been noted with VEE but is unlikely for the other two serotypes. Horse to horse spread of EEE is possible. Humans and horses are terminal hosts for WEE. Horses with WEE are sentinels for humans in a given area.
Highlands J virus, antigenically related to WEE virus, has been isolated in eastern USA. Although it is generally believed not to cause disease in mammals, it has been isolated from the brain of a horse dying of encephalitis in Florida (4).WEE virus infection in horses is often observed over a wide geographical area, e.g. sporadic cases over 1000 square miles. EEE virus infections are usually observed in limited geographical areas.
Seasonal Incidence
The disease is not directly contagious between horses and humans but occurs sporadically in both species from mid-summer to late autumn - during the height of the vector season. In temperate climates, case numbers peak in June to November. In warm climates, where the vector season is longer, the disease period is prolonged. Global warming may promote more cold climate outbreaks.
Epidemics
Prerequisites for epidemics to occur include adequate and adjacent numbers of reservoir animals, sufficient quantities of virulent virus, infected intermediate hosts, insect vectors and susceptiple horse and human populations. Outbeak prediction has been inaccurate, implyingf that other, unidentified factors may be important.
Pathogenesis
After inoculation into an equine host, viruses multiply in the muscle, enter the lymphatic circulation and localize in lymph nodes. Viral replication occurs in macrophages and neutrophils with subsequent shedding and significant clearance of viral particles. No further clinical signs develop if clearance is successful but neutralizing Abs are still produced. Viral immunological avoidance mechanisms include erythrocyte and leukocyte absorption. After incomplete elimination, residual virus infects endothelial cells and concentrates in highly vascular organs such as the liver and spleen. In these organs, viral replication produces circulating virus. The second viraemic period is typically associated with early clinical signs. CNS infection occurs within 3-5 days.
Viraemia during the acute phase of EEE and WEE. Incubation period of 1-3weeks after experimental infection with EEE or WEE. Incubtion often shorter with EEE. Central nervous system (CNS) replication within a week
3 key phases of the pathogenesis of alphavirus encephalitis. These are the early extraneural phase, the process of neuroinvasion itself, and virus and host factors related to neurovirulence.
Signalment
Unvaccinated adult horses and other equids are at risk in areas with suitable vectors. Vaccinated horses can still develop the disease, particularly if they are young or old.
Clinical Signs
The incubation of the disease after infection with the virus is from 1 to 3 weeks. In the initial stage there is fever, which may be accompanied by depression, and loss of appetite, but the reaction may be so mild it goes unnoticed. The virus causing Eastern Equine Encephalomyelitis (EEE) is the most virulent of the three types and the symptoms produced are the most severe, with a case fatality rate of up to 90%. The viraemia (level of virus in the blood) may be so high with this strain that horse to mosquito to horse cycling can occur. The nervous signs, when they appear, are hypersensitivity to sound and touch with periods of excitement and restlessness with apparent blindness. Affected horses may walk blindly into objects or walls. Muscle twitchings may occur in the face and shoulder muscles. A period of severe depression follows. Affected horses stand with their heads hung low and may have a half-chewed mouthful of feed hanging from their lips. The animal appears to be asleep and is unable to hold up his head and often rests it on some solid object. Although VEE does not cause as high a mortality as WEE/EEE, the clinical signs are similar. However, a second generalised infection may be caused by the virus causing fever, depression, colic and diarrhoea.
Worse in unvaccinated animals. Acute signs of EEE and WEE are nonspecific, last up to 5 days and include:
- mild to severe pyrexia
- anorexia
- stiffness
Early signs transient and often missed:
- pyrexia
- mild depression
Disease progression occurs more frequently with EEE than WEE:
- fever may rise and fall sporadically
Cerebral signs often occur a few days post-infection (but can occur at any time. In the acute phase the following may be noted:
- propulsive walking
- depression
- somnolence
- hyperaesthesia
- agression
- excitability
- frenzy in response to sensory stimulation
- conscious proprioceptive deficits
With progression, worsening cerebral cortical and cranial nerve dysfunction may result in:
- head pressing
- propulsive walking
- blindness
- circling
- head tilt
- facial and appendicular muscle fasciculations
- paralysis of pharynx, larynx and tongue
- recumbency for 1-7 days followed by death
VEE may have similar or different clinical presentations to WEE and EEE, which may relate to a persistently hightitre viraemia with VEE and differences in strain pathogenicity:
- pyrexia peaks early and remains high throuhgout the disease course
- mild fever and leukopenia associated experimentally with endemic strains
- severe pyrexia and leukopenia associated with epidemic strains
- diarrhoea, severe depression recumbency and death may precede neurological signs
- neurological signs around 4 days post-infection
- other associated signs: abortion, oral ulceration, pulmonary haemorrhage, epistaxis
- Paralysis of the lips
- Drooping eyelids
- Incoordination
Diagnosis
Presumptive based on clinical signs and epidemiological features. Definitive diagnosis requires serological tests and/or post-mortem examination. Virus isolation can be performed from blood or spinal fluid samples
Laboratory Tests
A combination of complement fixation (CF), haemagglutination inhibition (HAI) and cross-serum neutralization assays supports the acquisition of a positive diagnosis. A 4-fold increase in antibody (Ab) titre in convlescent sera is quoted for diagnosis but this test lacks sensitivity. The presence of viral Abs within 24hours of the initial viraemia typically precedes clinical signs. Ab titre increases sharply then deteriorates over 6 months. Samples taken when clinical signs appear are likely to miss the Ab peak and may thus demonstrate a decreasing titre. A single sample demonstrating an increased titre using HAI, CF and neutralizing Ab can provide a presumptive diagnosis.
Viral-specific IgM to the surface glycoprotein of Venezuelan EEV may be detected by ELISA, from 3 days post-onset of clinical signs up to 21 days post-infection. The ELISA is useful in acute VEE infections where convalescent serum samples are unobtainable. Viral culture may also be useful for acute VEE. Virus may be isolated from the CSF of acutely infected horses. Virus may be found in brain tissue using fluorescent Ab, ELISA and virus isolation.
Maternal-derived Ab may interfere with diagnosis in foals. The serum half-life of colostral Ab in foals is around 20days.
The virus is identified by complement fixation (CF), immunofluorescence, or plaque reduction neutralisation (PRN) tests. EEE and WEE viral RNA may also be detected by reverse-transcription polymerase chain reaction methods 1. Identification of the agent
The most definitive method for diagnosis of EEE or WEE is the isolation of the viruses. EEE virus can usually be isolated from the brains of horses, unless more than 5 days have elapsed between the appearance of clinical signs and the death of the horse. EEE virus can frequently be isolated from brain tissue even in the presence of a high serum antibody titre. WEE virus is rarely isolated from tissues of infected horses. Brain is the tissue of choice for virus isolation, but the virus has been isolated from other tissues, such as the liver and spleen. It is recommended that a complete set of these tissues be collected in duplicate, one set for virus isolation and the other set in formalin for histopathological examination. Specimens for virus isolation should be sent refrigerated if they can be received in the laboratory within 48 hours of collection; otherwise, they should be frozen and sent with dry ice. A complete set of tissues will allow the performance of diagnostic techniques for other diseases. For isolation, a 10% suspension of tissue is prepared in phosphate buffered saline (PBS), pH 7.8, containing bovine serum albumin (BSA) (fraction V; 0.75%), penicillin (100 units/ml), and streptomycin (100 µg/ml). The suspension is clarified by centrifugation at 1500 g for 30 minutes.
The newborn mouse is considered to be a sensitive host system. Inoculate intracranially one or two litters of 1-4-day-old mice with 0.02 ml of inoculum using a 26-gauge 3/8 inch (9.3 mm) needle attached to a 1 ml tuberculin syringe. The inoculation site is just lateral to the midline into the midportion of one lateral hemisphere. Mice are observed for 10 days; dead mice are collected daily and frozen at -70°C. Mouse brains are harvested for virus identification by aspiration using a 20-gauge 1 inch (2.5 cm) needle attached to a 1 ml tuberculin syringe. A second passage is made only if virus cannot be identified from mice that die following inoculation.
The chicken embryo is considered to be less sensitive than newborn mice when used for primary isolation of EEE and WEE viruses. Tissue suspensions can be inoculated by the yolk-sac route into 6-8-day-old embryonating chicken eggs. There are no diagnostic signs or lesions in the embryos infected with these viruses. Inoculated embryos should be incubated for 7 days, but deaths usually occur between 2 and 4 days post-inoculation. Usually only one passage is made unless there are dead embryos from which virus cannot be isolated. Newly hatched chickens are susceptible and have been used for virus isolation. If this method is used, precautions must be taken to prevent aerosol exposure of laboratory personnel, as infected birds can shed highly infectious virus.
EEE and WEE viruses can also be isolated in a number of cell culture systems. The most commonly used cell cultures are primary chicken or duck embryo fibroblasts, continuous cell lines of African green monkey kidney (Vero), rabbit kidney (RK-13), or baby hamster kidney (BHK-21). Isolation is usually attempted in 25 cm2 cell culture flasks. Confluent cells are inoculated with 1.0 ml of tissue suspension.
Following a 1-2-hour absorption period, maintenance medium is added. Cultures are incubated for 7 days, and one blind passage is made. EEE and WEE viruses will produce a cytopathic change in cell culture. Cultures that appear to be infected are frozen. The fluid from the thawed cultures is used for virus identification.
When the complement fixation (CF) test is used, EEE or WEE viruses can be identified in infected mouse or chicken brains, cell culture fluid, or amnionic-allantoic fluid. A 10% brain suspension is prepared in veronal (barbitone) buffer; egg and cell culture fluids are used undiluted or diluted 1/10 in veronal buffer. The fluid or suspension is centrifuged at 9000 g for 30 minutes, and the supernatant fluid is tested against hyperimmune serum or mouse ascitic fluid prepared against EEE and WEE viruses using a standard CF procedure (13). The CF test requires the overnight incubation at 4°C of serum-antigen with 7 units of complement. Virus can be identified in cell culture by direct immunofluorescent staining. The less commonly used method of virus identification is the neutralisation test, as outlined below.
EEE virus nucleic acid in mosquitoes and tissues has been identified by the polymerase chain reaction (PCR) using primers selected from the capsid gene (15). RNA is extracted using guanidium iso-thiocyanate-acid phenol. Forty repetitions of the three-step amplification cycle of nucleic acid denaturisation, primer annealing and primer extension are used. Temperature and duration of each step are optimised for the specific primer pair, reagents and thermal cycler used in the PCR cycles. Reaction products or their fragments are analysed on 2.0-2.6% agarose gels that have been stained with 1 µg/ml of ethidium bromide. An alternate identification procedure is by hybridisation with an oligonucleotide probe. A reverse-transcription PCR method for detection of WEE RNA and alternative methods for EEE RNA detection have also been described (5, 7).
Antigen-capture enzyme-linked immunosorbent assay (ELISA) has been developed for EEE surveillance in mosquitoes. This can be used in countries that do not have facilities for virus isolation or PCR (1).
2. Serological tests
Serological confirmation of EEE or WEE virus infection requires a four-fold or greater increase or decrease in antibody titre in paired serum samples collected 10-14 days apart. Most horses infected with EEE and WEE virus have a high antibody titre when clinical disease is observed. Horses infected with EEE or WEE virus usually have antibody titres in the acute stage of the disease. Consequently, a presumptive diagnosis can be made if an unvaccinated horse with appropriate clinical signs has antibody against only EEE or WEE virus. The detection of IgM antibody by the ELISA can also provide a presumptive diagnosis of acute infection (11). The plaque reduction neutralisation (PRN) test or, preferably, a combination of PRN and haemagglutination inhibition (HI) tests is the procedure most commonly used for the detection of antibody against EEE and WEE viruses. There are cross-reactions between antibody against EEE and WEE virus in the CF and HI tests. CF antibody against both EEE and WEE viruses appears later and does not persist; consequently, it is less useful for the serological diagnosis of disease.
a) Complement fixation
The CF test is frequently used for the demonstration of antibodies, although the antibodies detected by the CF test may not persist for as long as those detected by the HI or PRN tests. A sucrose/acetone mouse brain extract is commonly used as antigen. The positive antigen is inactivated by treatment with 0.1% beta-propiolactone.
In the absence of an international standard serum, the antigen should be titrated against a locally prepared positive control serum. The normal antigen, or control antigen, is mouse brain from uninoculated mice similarly extracted and diluted.
Sera are diluted 1/4 in veronal buffered saline containing 1% gelatin (VBSG), and inactivated at 56°C for 30 minutes. Titrations of positive sera may be performed using additional twofold dilutions. The CF antigens and control antigen (normal mouse brain) are diluted in VBSG to their optimal amount of fixation as determined by titration against the positive sera; guinea-pig complement is diluted in VBSG to contain 5 complement haemolytic units-50% (CH50). Sera, antigen, and complement are reacted in 96-well round-bottom microtitre plates at 4°C for 18 hours. The sheep red blood cells (SRBCs) are standardised to 2.8% concentration. Haemolysin is titrated to determine the optimal dilution for the lot of complement used. Haemolysin is used to sensitise 2.8% SRBCs and the sensitised cells are added to all wells on the microtitre plate. The test is incubated for 30 minutes at 37°C. The plates are then centrifuged (200 g), and the wells are scored for the presence of haemolysis. The following controls are used: (a) serum and control serum each with 5 CH50 and 2.5 CH50 of complement; (b) CF antigen and control antigen each with 5 CH50, and 2.5 CH50 of complement; (c) complement dilutions of 5 CH50, 2.5 CH50, and 1.25 CH50; and (d) cell control wells with only SRBCs and VBSG diluent. These controls test for anticomplementary antigen, anticomplementary serum, activity of complement used in the test, and integrity of the SRBC indicator system in the absence of complement, respectively.
To avoid anticomplementary effects, sera should be separated from the blood as soon as possible. Positive and negative control sera should be used in the test.
b) Haemagglutination inhibition
The antigen for the HI test is the same as described above for the CF test. The antigen is diluted so that the amount used in each haemagglutinating unit (HAU) is from four to eight times that which agglutinates 50% of the RBCs in the test system. The haemagglutination titre and optimum pH for each antigen are determined with goose RBCs diluted in pH solutions ranging from pH 5.8 to pH 6.6, at 0.2 intervals.
Sera are diluted 1/10 in borate saline, pH 9.0, and then inactivated at 56°C for 30 minutes. Kaolin treatment is used to remove nonspecific serum inhibitors. Sera should be absorbed before use by incubation with a 0.05 ml volume of washed packed goose RBCs for 20 minutes at 4°C.
Following heat inactivation, kaolin treatment and absorption, twofold dilutions of the treated serum are prepared in borate saline, pH 9.0 with 0.4% bovalbumin. Serum dilutions (0.025 ml/well) are prepared in a 96-well round-bottom microtitre plate in twofold dilutions in borate saline, pH 9.0, with 0.4% bovalbumin. Antigen (0.025 ml/well) is added to the serum. Plates are incubated at 4°C overnight. RBCs are derived from normal white male geese (RBCs from adult domestic white male geese are preferred, but RBCs from other male geese can be used. If cells from female geese are used, there may be more test variability. It has been reported that rooster RBCs cause a decrease in the sensitivity of the test) and washed three times in dextrose/gelatin/veronal (DGV), and a 7.0% suspension is prepared in DGV. The 7.0% suspension is then diluted 1/24 in the appropriate pH solution, and 0.05 ml per well is added immediately to the plates. Plates are incubated for 30 minutes at 37°C. Positive and negative control sera are incorporated into each test. A test is considered to be valid only if the control sera give the expected results. Titres of 1/10 and 1/20 are suspect, and titres of 1/40 and above are positive.
c) Enzyme-linked immunosorbent assay
The ELISA is performed by coating flat-bottomed plates with anti-equine IgM capture antibody (11). The antibody is diluted according to the manufacture's recommendations in 0.5 M carbonate buffer, pH 9.6, and 50 µl is added to each well. The plates are incubated at 37°C for 1 hour, and then at 4°C overnight. Prior to use, the coated plates are washed twice with 200 µl/well of 0.01 M PBS containing 0.05% Tween 20. After the second wash, 200 µl/well of PBS/Tween/5% nonfat dried milk is added and the plates are incubated at room temperature for 1 hour. Following incubation, the plates are washed again three times with PBS/Tween. Test and control sera are diluted 1/100 and 1/1000 in 0.01 M PBS, pH 7.2, containing 0.05% Tween 20, and 50 µl is added to each well. The plates are incubated at 37°C for 2 hours and then washed three times. Next, 50 µl of viral antigen is added to all wells. (The dilution of the antigen will depend on the source and should be empirically determined.) The plates are incubated overnight at 4°C, and washed six times. Then, 50 µl of horseradish-peroxidase-conjugated monoclonal antibody (MAb) to encephalitis virus (available from: Centers for Disease Control and Prevention, Biological Reference Reagents, 1600 Clifton Road NE, Mail Stop C21, Atlanta, Georgia 30333, United States of America) is added. The plates are incubated for 60 minutes at 37°C and then washed three times. Finally, 50 µl of freshly prepared ABTS (2,2'-Azino-bis-[3-ethylbenzo-thiazoline-6-sulphonic acid]) substrate + hydrogen peroxidase is added, and the plates are incubated at room temperature for 15-40 minutes The absorbance of the test serum is measured at 405 nm. A test sample is considered to be positive if the absorbance of the test sample in wells containing virus antigen is at least twice the absorbance of negative control serum in wells containing virus antigen and at least twice the absorbance of the sample tested in parallel in wells containing control antigen.
d) Plaque reduction neutralisation
The PRN test is very specific and can be used to differentiate between EEE and WEE virus infections. The PRN test is performed in duck embryo fibroblast, Vero, or BHK-21 cell cultures. The sera can be screened at a 1/10 and 1/100 final dilution. Endpoints can be established using the PRN or HI test. Serum used in the PRN assay is tested against 100 plaque-forming units of virus. The virus/serum mixture is incubated at 37°C for 75 minutes before inoculation on to confluent cell culture monolayers in 25 cm2 flasks. The inoculum is adsorbed for 1 hour, followed by the addition of 6 ml of overlay medium. The overlay medium consists of two solutions that are prepared separately. Solution I contains 2 x Earle's Basic Salts Solution without phenol red, 6.6% yeast extract lactalbumin hydrolysate, 4% fetal bovine serum, 800 units/ml penicillin, 400 µg/ml streptomycin, 200 µg/ml nystatin, 6% of a 7.5% solution of sodium bicarbonate, and 3.3% of a 1/1500 dilution of neutral red (1/8000). Solution II consists of 2% Noble agar that is sterilised and maintained at 47°C. Equal volumes of solutions I and II are adjusted to 47°C and mixed together just before use. The test is incubated for 48-72 hours, and endpoints are based on a 90% reduction in the number of plaques compared with the virus control flasks, which should have about 100 plaques.
Clinical Pathology
Changes in cerebrospinal fluid (CSF) include increased cellularity (50-400 mononuclear cells per microlitre) and protein concentration (100-200mg/dl)
Post-mortem findings
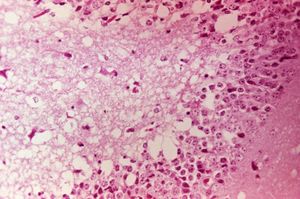
PRECAUTION: infective viral particles may be present in CNS and other tissues. The brain and spinal cord are typically grossly normal, but vascular congestion and discolouration of the CNS may be seen. Histologically the entire brain is affected by nonseptic mononuclear cell and neutrophilic inflammation. Severe lesions are noted in the cerebral cortex, thalamus and hypothalamus. Mononuclear meningitis, neuronal degeneration, gliosis and perivascular cuffing with mononuclear cell and neutrophilic infiltration are evident. Immunohistochemistry can be diagnostic. Liquefactive necrosis and haemorrhage of the cerebral cortex, atrophy of the pancreatic acinar cells and hyperplasia of the pancreatic duct cells commonly occur with VEE.
IN VEE there may be damage to other organs such as the pancreas, liver and heart.
Gross pathological lesions are rarely observed in horses and, if present, consist only of the congestion of the brain and meninges. Ecchymotic haemorrhages of traumatic origin may be observed. Microscopic lesions are usually found throughout the central nervous system and can be diagnostic. There is widespread evidence of a severe inflammatory response involving the grey matter. Neuronal degeneration with infiltration by polymorphonuclear leukocytes, diffuse and focal gliosis, and perivascular cuffing with lymphocytes and neutrophils are seen. Also observed are neuronophagia and liquefaction of the neuropil. The extent of the lesions depends on the severity of the infection and the duration of the neurological involvement (16). Brain lesions caused by WEE virus infection are focal and have lymphocytic infiltrations. Brain lesions caused by EEE virus infection are more severe and are found throughout the grey matter. They are characterised by a larger number of neutrophils among the inflammatory cells.
Differential diagnosis
- Other togaviral encephalitides
- Trauma
- Hepatic encephalopathy
- Rabies
- Leukoencephalomalacia
- Bacterial meningoencephalitis
- Equine protozoal myeloencephalitis (EPM)
- Verminous encephalomyelitis
- West Nile Virus (WNV) infection
- Toxicosis
Treatment
No effective, specific treatment is available. Supportive management includes:
- NSAIDs (phenylbutazone, flunixin meglumine) to control pyrexia, inflammation and discomfort
- DMSO IV in a 20% solution to control inflamation, provide some analgesia and mild sedation
- Pentobarbital, diazepam IV, phenobarbital PO or phenytoin IV to control convulsions
- Antibiotic therapy in cases with secondary bacterial infection
- Balanced fluid solutions IV or PO as necessary to correct hydration status
- Dietary supplementation (enteral or parenteral if anorexia persists more than 48 hours)
- Laxatives to minimize the risk of impaction
- Protection of areas susceptible to self-induced trauma and provision of deep bedding
- Sling support if the horse is recumbent
Prognosis
Comatose animals rarely survive. Survivors exhibit functional improvement over weeks to months, but complete recovery from neurological deficits is rare. Residual ataxia, depression and abnormal behaviour is often seen with EEE, less commonly with WEE. The mortality rates for neurological equine viral encephalitis are reportedly:
- EEE 75-100%
- WEE 20-50%
- VEE 40-80%
It is generally assumed that infection does not provide protective immunity, however, protection for up to 2 years has been noted.
Control
Vaccination
Vaccinate susceptible horses annually. Vaccinate horses in the face of an outbreak. Vaccinate mares one month prior to foaling. Colostral-derived Ab persists for 6-7 months. Although folas ca be vaccinated at any time, they should be re-vaccinated at 6 months and at one year if they were vaccinated early. Most vaccines are killed (produced in cell culture and inactivated with formalin) and elicit significant increases in Ab titre after 3 days. Protective titres last for 6-8 months. Some cross-protection is seen between the serotypes but not between WEE and EEE. Monovalent, divalent and trivalent vaccines are available but the response to VEE vaccination alone is decreased in horses previously vaccinated against WEE and EEE. Susceptible horses should be vaccinated annually in late spring or several months before the high risk season. Biannual or triannual vaccination is recommended in regions where the mosquito season is prolonged. Vaccination does not interfere with the ELISA assay for VEE. PRECAUTION: human vaccination recommended for vets in endemic areas.
Vector control
Responsible use of insecticides and repellents, elimination of standing water and stable screening will all help to reduce viral transmission. Environmental application of insecticides may be useful in endemic areas or during an outbreak. Horses infected with Venezuelan EEV should be isolated for 3 weeks after complete recovery.
References
Bertone, J.J (2010) Viral Encephalitis in Reed, S.M, Bayly, W.M. and Sellon, D.C (2010) Equine Internal Medicine, Third Edition, Saunders, Chapter 12.
Manual of Diagnostic Tests and Vaccines for Terrestrial Animals http://www.oie.int/eng/normes/mmanual/A_00081.htm 4. Karabatsos N., Lewis A.L., Calisher C.H., Hunt A.R. & Roehrig J.T. (1988). Identification of Highland J virus from a Florida horse. Am. J. Trop. Med. Hyg., 39, 603-606. Walton T.E. (1981). Venezuelan, eastern, and western encephalomyelitis. In: Virus Diseases of Food Animals. A World Geography of Epidemiology and Control. Disease Monographs, Vol. 2, Gibbs E.P.J., ed. Academic Press, New York, USA, 587-625.
http://www.defra.gov.uk/foodfarm/farmanimal/diseases/atoz/viralenceph/index.htm
Vet Pathol. 2010 Jun 15. [Epub ahead of print] Review Paper: Pathology of Animal Models of Alphavirus Encephalitis. Steele KE, Twenhafel NA.
| Also known as: | Alphaviral encephalitis, Alphaviral encephalitides Eastern equine encephalitis, Eastern equine encephalomyelitis, EEE |