Difference between revisions of "Fasciolosis"
| Line 58: | Line 58: | ||
===Pathology=== | ===Pathology=== | ||
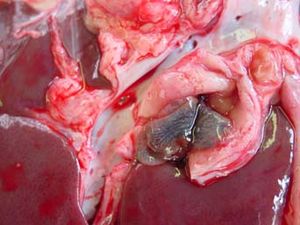
| − | [[Image:Fasciola hepatica - bile duct.jpg| | + | [[Image:Fasciola hepatica - bile duct.jpg|300px|thumb|right|Bile duct fibrosis due to chronic ''Fasciola hepatica'' infestation. Source: Wikimedia Commons; author: Flukeman]] |
The pathology of fasciolosis is similar in both sheep and cattle, although the species have a different incidence of acute and chronic disease, as described above. | The pathology of fasciolosis is similar in both sheep and cattle, although the species have a different incidence of acute and chronic disease, as described above. | ||
Revision as of 18:35, 5 August 2010
| This article is still under construction. |
| Also known as: | Fascioliasis Fasciolasis Fluke |
Description
Fasciolosis is a condition of ruminants which causes subclinical and clinical disease leading to ill thrift and deaths. The causative organism is the trematode Fasciola hepatica which primarily parasitises the bile ducts of sheep and cattle but may occasionally be found in the horse. Lymnaea truncatula, a mud snail, is the intermediate host of Fasciola hepatica, and transmission of disease is dependent on the presence of appropriate snail habitats. These habitats are more plentiful in areas of high rainfall, such as the western British Isles. However, infected animals may be found outwith these areas due to the transportation of livestock, or unusual weather patterns. The association of fasciolosis with wetter habitats lends a seasonal nature to disease outbreaks, and can help predict the severity of these.
For instance, wet summers increase both the number of snail habitats and the hatching of fluke eggs, leading to many infected snails. These in turn shed many cercariae, which form a high density of metacercariae on herbage to increase the risk of fasciolosis. Conversely, in dry summers, fewer fluke eggs hatch and snails are restricted to their permanent habitats. Fewer snails become infected and cercariae and metacercariae numbers are low and confined to the areas where snails can survive. The risk of fasciolosis is therefore reduced.
Upon ingestion, metacercariae excyst to present as immature flukes in the small intestine. These then migrate across the peritoneal cavity over a period of roughly one week, and invade the liver. Larvae continue to migrate within the hepatic parenchyma, beoming more destructive as they grow to a length of up to one centimetre. In about six weeks, immature fluke enter the bile ducts and mature to adults, where they begin to produce eggs. The prepatent period is around ten to twelve weeks.
In sheep, "acute" disease caused by fluke larvae is the most common presentation, and generally occurs in the wetter Autumn and early Winter months in both lambs and ewes. Fasciolosis in cattle can occur at any time of year and tends to involve adult fluke, causing "chronic" disease.
A slightly larger trematode, F. gigantica, causes a similar condition in tropical regions.
Signalment
Fasciolosis affects both young and adult animals, primarily sheep and cattle.
Diagnosis
A diagnosis can usually be made using clinical findings and the seasonal occurance of disease, and may be supported by a history of fasciolosis on the farm and post-mortem findings. Certain adjunctive tests may also prove useful.
Clinical Signs
In sheep, fasciolosis may present as acute, chronic, or infrequently sub-acute manifestations. Acute fasciolosis usually occurs between September and December and is caused by large numbers of immature Fasciola hepatica migrating through the liver parenchyma and causing massive damage. It arises within around two to six weeks of ingestion of metacercariae. If sheep are not exposed to at-risk pasture until later in the year, acute fasciolosis may occur as late as the following Feburary. Hepatic damage caused by migration of fluke larvae gives clinical signs including lethargy, pallor, dyspnoea and death in both young and adult animals. Handling of sheep may cause liver rupture and sudden death, and sudden death may also occur due to Black's disease (Clostridium novyi type B) or bacillary haemoglobinuria (Clostridium novyi type D) in unvaccinated sheep. This is a result of necrosis caused by larval migration within the liver: anaerobis conditions are created, enabling multiplication of clostridial organisms and thus toxin production.
Chronic fasciolosis in sheep is caused by adult flukes in the bile ducts and is usually seen in February and March, 4-5 months after ingestion of metacercariae. However, cases may present in early summer if snails become infected during the winter. Progressive weight loss over weeks to months results in poor body condition, and anorexia is often seen. As adult flukes feed on blood and are capable of consuming 0.5ml each per day, anaemia and pallor frequently occur in chronic fasciolosis. Initially, this regenerative anaemia is normochromic, but becomes hypochromic as iron reserves are depleted. Hypoalbuminaemia may also result from whole blood loss, and from reduced hepatic production. This gives a reduced plasma oncotic pressure, leading to ascites and/or submandibular oedema in advanced cases.
In some cases, sub-acute fasciolosis may occur if infections has occured over a prolonged period. In these instances, disease is caused by both adult flukes and larvae and ill thrift, lethargy, dyspnoea is seen from around December to March.
In addition to these presentations, Fasciola hepatica has subclinical effects on sheep. Fleece weight and fibre quality are affected by even small fluke burdens, and there is some evidence that lambing percentage and lamb growth rates may be negatively influenced. Condemnation of affected livers at slaughter also causes economic losses.
In cattle, fasciolosis is usually a disease of calves occuring between winter and spring, but may affect any animal at any time of year. Disease is usually chronic (caused by adult flukes) and signs tend to be less severe than in sheep. Poor nutrition and gastrointestinal parasitism do however exacerbate disease. As in sheep, subclinical losses include liver condemnation following slaughter and extended finishing times. Carcass values and milk yield may be reduced in beef and dairy cattle respectively. Milk quality may also be poorer in Fasciola hepatica infection.
Laboratory Tests
A simple and effective test for Fasciola hepatica infection is examination of the faeces for fluke eggs. These are large, ovoid and golden brown, but may be few in number and thus difficult to find. It must also be remembered that eggs are produced only by adult flukes, and so will not be detectable in acute disease.
Measurement of serum glutamate dehydrogenase (GLDH) and/or glutamyl transpeptidase (GGT) levels may prove useful. GLDH is an enzyme released by damaged hepatic cells and becomes elevated within the first few weeks of infection. GGT indicates damage to the epithelial cells lining the bile ducts and rises later in disease, although high levels are maintained for longer. Fluke serology is possible by means of a reliable ELISA test that identifies anti-fluke antibodies in the serum or in milk.
Biopsy
Biopsy or histopathology may reveal necrotic areas of liver, haemorrhage, hyperplastic bile ducts or indeed the presence of Fasciola hepatica within the section.
Pathology
The pathology of fasciolosis is similar in both sheep and cattle, although the species have a different incidence of acute and chronic disease, as described above.
In acute fasciolosis, immature flukes grow and migrate within the liver parenchyma, causing necrotic tracts and haemorrhage. On post-mortem, the liver is enlarged and a fibrinous peritonitis may be be present. Fibrin takes are often particularly apparent on the ventral lobe. Histologically, tracts are seen as areas of degenerate hepatocytes and haemorrhage, which may later become infiltrated with eosinophils and lymphocytes. In the long term, these areas will become fibrosed. In sub-acute fasciolosis, the liver again is enlarged on post-mortem, and haemorrhagic tracts can be seen.
Chronic fasciolosis is associated with damage to the bile ducts by adult flukes. On post-mortem, the liver is distorted by areas of fibrosis caused by the migration of the original immature flukes. The bile ducts are dilated, and flukes may be expressed from within. The gall bladder may also be enlarged. The walls of the bile ducts may be ulcerated and haemorrhagic with areas of epithelial hyperplasia. The walls eventually become fibrosed and may calcify in cattle. Calcified bile ducts can be seen protruding from the liver surface - this is known as "pipe stem liver".
Treatment
Forecasting Fasciolosis
Several prediction models have been developed. They evaluate the wetness of the soil from May to October by taking account of rainfall patterns and evapo-transpiration. Seasonal weighting factors are applied. June is a particularly influential month.
A drought in late summer can reverse a potentially dangerous trend, and so unqualified forecasts should not be issued prematurely.
A high snail density will only lead to disease if infection is present (i.e. if fluke eggs have been deposited onto the habitat by farm or wild animals). Local biological interpretation of computer generated predictions is therefore required.
These forecasting models can provide valuable information for the farming community. MAFF (before DEFRA) used to provide an annual forecast, but this is now discontinued.
Control
Anthelmintics
- Few flukicides kill all parasitic developmental stages
- Not all products, therefore, are suitable for controlling acute outbreaks
- The anthelmintic with the broadest spectrum of activity against immature and adult F. hepatica is Triclabendazole
- Triclabendazole-resistant F. hepatica populations are beginning to emerge
- The more potent products tend to be the most expensive
- Many flukicides bind to plasma protein and have long plasma half-lives
Chemoprophylaxis
There are several control objectives
- To prevent fluke eggs being dropped onto snail habitats
- This is done by treating sheep/cattle with an adulticidal drug in the late winter/early spring
- To protect animals grazing pasture known to be contaminated with metacercariae. The choice of drug, time of treatment and dosing interval will depend on
- Whether you are trying to prevent acute or chronic disease
- The likely intensity of challenge (local knowledge/fluke forecast)
- The persistent effect of the drug (i.e. the period after dosing, during which the animal is protected from reinfection)
Vaccination
- A recombinant vaccine providing approximately 70% protection for cattle is being developed
- It exerts its effect by stimulating a range of immune responses not normally seen in chronically infected animals (including TH1-type responses)
Molluscicides
- These have been employed with success in the past, but are no longer used. This is because they have to be applied before any fluke forecast can be issued. (Farmers are unwilling to invest in control measures before they are known to be necessary). Also, they have to be applied very carefully as snails can rapidly recolonise sprayed land if any habitat has been missed
Alternative strategies
- An ability to recognise and define the extent of snail habitats allows alternative cost-effective control options such as fencing and drainage
Treatment
If the fuke is present treat with triclabendazole, which is effective against all stages of Fasciola hepatica. Treatment should be applied in September/October and again in January, if faecal egg count is still postitive. One may also treat against adult only stages in May/June, preventing any future pasture contamination. However, do not use the same treatment in September/October as used in May/June, as resistance to drugs is becoming a real problem within the UK due to overuse. If it has been a particularly wet season, it may be necessary to treat again, as Fasciola hepatica becomes more prevalent under such conditions.
Isolation and treatment of all new animals entering from another farm has also be shown to be effective. Other control measures include fencing off wet areas, and increasing soil drainage.