Pulpy Kidney
| This article is still under construction. |
Description
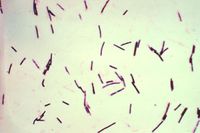
Pulpy kidney is a common, peracute and usually fatal enterotoxaemia of sheep of all ages, caused by the ε toxin of Clostridium perfringens type D. C. perfringens is a large, gram positive, anaerobic bacillus that is ubiquitous in the environment and commensalises the gastrointestinal tract of most mammals1, although type D is found less abundantly in healthy animals than the other genotypesivis. Five genotypes of Clostridium perfringens exist, named A-E, and all genotypes produce potent exotoxins. There are 12 exotoxins in total, some of which are lethal and others which are of minor significance2. These are produced as pro-toxins, and are converted to their toxic forms by digestive enzymes such as trypsin. The enterotoxaemias are a group of diseases caused by proliferation of C. perfringens in the lumen of the gastrointestinal tract and excessive production of exotoxin.
In healthy animals, there is a balance between multiplication of Clostridium perfringens and its passage in the faeces. This ensures that infection is maintained at a low level. However, C. perfringens is saccharolytic and is therefore able to multiply rapidly when large quantities of fermentable carbohydrate are introduced to the anaerobic conditions of the abomasum and small intestine, leading to build-up of exotoxin. Gut statis, for example due to insufficient dietray fibre or a high gastrointestinal parasite burden, can also contribute to the accumulation of toxins.
Enterotoxaemia due to Clostridium perfringens type D causes sudden death in sheep of any age, particularly well-grown lambs of between 4 and 10 weeks of age and fattening lambs of 6 months to 1 year oldlewis. Rams are also susceptible when they are subjected to an incraesed plane of nutrition prior to mating. The condition is associated with a change in diet, for example to include lush grass or high proportions of concentrate. This leads to rapid multiplication of the bacterium and excessive production of its ε toxin. ε toxin causes loss of epithelial integrity, leading to increased capiliary permeability and oedema formation in many tissues, particularly the brainmerck, ivis. The incidence of pulpy kidney declined over the past 25 years or so, due to the widespread use of clostridial vaccinessargison, but the condition is now becoming a problem again as complacency reduces the use of vaccination. At its most extreme, pulpy kidney can cause losses of 10-15% of the lamb crop. The disease can also occur in cattle, but this is rareivis.
Signalment
Pulpy kidney can affect unvaccinated sheep of all ages. However, it is most common in 4-10 week old lambs, and fattening lambs between 6 months and 1 year of age.
Diagnosis
A presumptive diagnosis of pulpy kidney can be made based on a history of sudden deaths in lambs recently introduced to carbohydrate-rich feed. If animals are seen before they die, certain clinical signs may be suggestive of pulpy kidney, and post-mortem examination may also aid diagnosis. Laboratory tests support, but do not confirm, a diagnosis of pulpy kidney.
Clinical Signs
The first indication of pulpy kidney disease is the occurence of sudden death in the best-grown lambsmerck, lewis, ivis. Occasionally, animals may be seen alive displaying clinical signs suggestive of the condition. Signs are typically neurological and include hyperaesthesia, ataxia, circling or head-pressing, with rapid progression to recumbency, opisthotonus, convulsions and deathmerck, lewis, ivis. Diarrhoea may also be seen, and hyperglycemia or glycosuria is frequently reportedmerck, lewis.
Adult sheep are sometimes affected and show weakness, incoordination, and convulsions. Death occurs within 24 hoursmerck.
Laboratory Tests
Short, thick gram-positive rods are easily visualised on smears of intestinal contents. An ELISA may be used to demonstrate the presence of ɛ toxin in the small intestinal or peritoneal fluid. A positive toxin ELISA supports but does not confirm a diagnosis, since immune animals may experience elevated toxin levels without suffering ill effects. PCR can also be used to identify ɛ toxin indirectly by detecting its gene.
Pathology
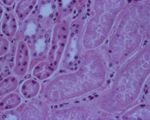
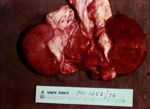
ivis At necropsy examination, the peritoneal, pleural, and / or pericardial spaces are filled with variable volumes of straw- or red-colored fluid that may contain fibrin clots. Petechial hemorrhages are often visible on the visceral surfaces. Pulmonary and mesenteric edema may be evident. Gross lesions of the intestinal tract are frequently absent in affected sheep. Dipstick analysis of urine collected from the bladder frequently reveals the presence of glucose. The renal cortex may be softened (hence the term "pulpy kidney"), although this is a nonspecific autolytic change seen on occasion in small ruminant cadavers. The thalamus and cerebellum may be appreciably soft, with scattered hemorrhages therein. Occasionally, no gross lesions are seen in ovine cases of type D enterotoxemia [24].
merck Necropsy may reveal only a few hyperemic areas on the intestine and a fluid-filled pericardial sac. This is particularly the case in young lambs. In older animals, hemorrhagic areas on the myocardium may be found as well as petechiae and ecchymoses of the abdominal muscles and serosa of the intestine. Bilateral pulmonary edema and congestion frequently occur but usually not in young lambs. The rumen and abomasum contain an abundance of feed, and undigested feed often is found in the ileum. Edema and malacia can be detected microscopically in the basal ganglia and cerebellum of lambs. Rapid postmortem autolysis of the kidneys has led to the popular name, pulpy kidney disease; however, pulpy kidneys are by no means always found in affected young lambs and are seldom found in affected goats or cattle. Hemorrhagic or necrotic enterocolitis may be seen in goats.
- Animals are very bloated.
- Often lack lesions.
- Guts appear slightly flaccid, and a bit hyperaemic.
- The toxaemic carcase has petechial and echymotic haemorrhages, and hydropericardium.
- The kidneys are characteristically affected.
- Become oedematous, mushy and red within about 1 hour of death.
- A stream of water on cut surface of the kidney washes away the epithelium (since it is necrotic) and leaves fronds of tissue (This is the case with any kidney if the post mortem is more than a few hours after death).
- This accelerated post mortem change is a characteristic of the disease.
- Glycosuria is a feature
- The toxin affects the epithelium of the proximal part of nephron, stopping resorption of glucose.
- Note: the swelling of cells in the kidney means that anuria occurs, meaning there is no urine avaialable to rest for glucose.
- It is sometimes possible to get a positive reaction to a stick test by wiping it on the inside of the bladder.
- Periportal hepatic fatty change occurs.
- This is only really seen histologically.
- Grossly, the liver looks "half cooked", but this is true of many bacterial diseases.
- Histopathology is often not helpful.
- Necrosis of cells in proximal convoluted tubules.
- There is also a characteristic degeneration in parts of the brain - focal symmetrical encephalomalacia
- Lesions due to effect of epsilon toxin on blood vessels.
Treatment and Control
Presentation of lamb dysentery is usually peracute, with sudden deaths occuring before treatment can be implemented. Even if animals are found prior to death, treatment is usually unrewarding as organs are irreversibly damaged by toxins by the time signs present2. Instead, a definitive diagnosis should be pursued before greater losses occur, and the farmer should be encouraged to submit the carcase for further investigations.
As treatment is so ineffective, much emphasis is put on to the control of lamb dysentery. Vaccination in the face of an outbreak has been shown to be effective7, and specific hyperimmune serum can also be administered4, 6t. Oral antibiotics may be given4 but are regarded as a less appropriate therepautic. Management measures such as removing the flock from a particular pasture or reducing concentrate feeding may be implemented in other clostridial diseases but are of no benefit in lamb dysentery: over-ingestion of the dam's milk combined with poor hygiene are responsible for this disease. Therefore, sufficient supervision should be given at lambing time to ensure adequate intakes of colostrium and the maintenance of good hygiene.
Lamb dysentery can be controlled through vaccination against clostridial diseases. Before the development of modern clostridial vaccines in the 1970s, catastrophic losses of up to 30% of the lamb crop could occur due to lamb dysentery2. The vaccines used today are effective against a variety of clostridial diseases and some vaccines are combined for effects against Pasteurella. The vaccines consist of toxoids which are inactivated forms of the toxins produced by clostridial organisms. The principles of vaccination are the same whether a clostridium-only or Pasteurella-combined product is used: a sensitising dose must be given 4-6 weeks before a second, confirming dose2. As immunity wanes over a period of a year booster doses are required annually. Therefore, ewes should receive the primary vaccination course before entering the breeding flock and an annual booster approximately six weeks before lambing. Timing the booster vaccination in this way affords passive protection to lambs until they are around sixteen weeks of age. Lambs born to unvaccinated ewes should be vaccinated between 3 and 12 weeks old, with a second injection given at least four weeks later.
Links
- The Merck Veterinary Manual: Pulpy Kidney
- The Center for Food Security and Public Health Animal Disease Factsheet: Epsilon toxin of Clostridium perfringens.
- NOAH: Vaccination of farm animals
- Clostridia.net - Clostridium perfringens