Blood Gas Analysis
Introduction
Blood gas analysis is the gold standard for evaluating gas exchange in a patient. Analysers measure the pH, partial pressures of oxygen and carbon dioxide of the sample directly as well as calculating other parameters such as haemoglobin saturation and plasma bicarbonate. It provides details on oxygenation, ventilation and acid-base status of the patient. Samples used are usually heparinised and can be arterial or venous, but this should be considered when interpreting results.
pH
- Direct measurement
- Normal values - 7.35 -7.45
- Increases indicate an alkalaemia.
- Decreases indicate an acidemia.
Acid / Base
It is essential that 50-100meq of acid is excreted by the kidneys every day. This is achieved by secretion of H+ in two regions of the nephron, the proximal tubule and the collecting ducts, and is essential for maintaining the acid base ratio, within the body, at the correct levels. If there is a net gain or loss of H+ within the body then the kidneys will compensate for it. The H+ ions cannot be secreted as free ions, however virtually all filtered HCO3- must be reabsorbed. The result is that the H+ ions bind to other filtered buffers which are not fully reabsorbed such as ammonia or phosphate. Extracellular pH is th main physiological regulator affecting how much acid is secreted. In pathological states circulating volume, aldosterone and plasma potassium affect it.
The Role of the Kidneys in Acid Base
The kidneys work with the respiratory system to regulate H+. Where as the respiratory systems quickly compensates for a problem it is left to the kidneys to actually remove the problem and restore a proper balance. They do this by altering the plasma concentration of HCO3-.
Alkalosis
In a situation of alkalosis the kidneys allow more HCO3- to be excreted. This results in an increase in an increase of un-buffered H+ and thus returns the pH towards normal.
Acidosis
In a situation of increased H+ levels the body is said to be in a state of acidosis and the kidneys stop excreting HCO3- and the tubular cells produce more bicarbonate. This results in more H+ being buffered and the pH increases back to normal
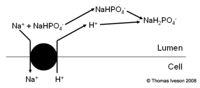
Buffers of H+ in Urine
- Weak acids are filtered and act as buffers
- Ability depends upon pKa+ and concentration
- Once all the bicarbonate has been reabsorbed the secreted H+ combine with these instead.
- The H+ ion is then excreted
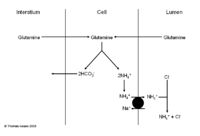
The Role of Ammonium in the Proximal Tubule
- The body is able to excrete H+ as ammonium NH4+. This is very useful as:
- It adds flexibility to renal acid base regulation and can help regulate NH4+
- It is ionised, fat insoluble and trapped therefore is excreted
- It is easily replaced so is quite a good method
- Under physiological control
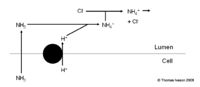
The Role of Ammonium in the Collecting Ducts
- Very different mechanism
- No bicarbonate produced
- See diagram
The Effect of The Buffers on Body pH
- When H+ is binding to buffers other than bicarbonate it is excreted. This means that the reabsorption of bicarbonate represents a net gain of bicarbonate not just a replacement of what was filtered. This results in an increase in plasma pH.
- Bicarbonate is also produced as part of the secretion of ammonium in the proximal tubule
Regulation
- H+ is important for both HCO3- reabsorption and generation of new HCO3-
- However the rate of secretion must be carefully regulated
- There must be enough H+ secreted to reabsorb all the filtered HCO3-
Oxygen
Partial Pressure
- Direct measurement
- Assuming the inspired oxygen fraction is 21%, normal values are 80-100mmHg (10.7-13.3kPa)
- Increases may be due to increases in fractional inspired oxygen or atmospheric pressure.
- Decreases indicate hypoxaemia if below 60mmHg (8kPa)
Saturation
- Calculated value.
- Normal values - 95-100%
- Decreases indicate hypoxaemia if below 90%
Carbon Dioxide
Partial Pressure
- Direct measurement
- Normal values - 35-45mmHg
- Increases indicate a respiratory acidosis.
- Decreases indicate a respiratory alkalosis.
Plasma Bicarbonate
- Calculated value.
- Normal values - 22-26 mmol/l
- Increases indicate a metabolic alkalosis.
- Decreases indicate a metabolic acidosis.