Coagulation Tests
| This article is still under construction. |
Also known as: coagulation profile, clotting profile, clotting tests.
Introduction
Haemostasis
Normally, haemostastis is maintained by three key events. The first stage, primary haemostasis, involves platelets and the blood vessels themselves. It is triggered by injury to a vessel, and platelets become activated, adhere to endothelial connective tissue and aggregate with other platelets. A fragile plug is thus formed which helps to stem haemorrhage from the vessel. Substances are released from platelets during primary haemostasis. Vasoactive compounds give vasoconstriction, and other mediators cause continued platelet activation and aggregation, as well as contraction of the platelet plug. Primary haemostasis ceases once defects in the vessels are sealed and bleeding stops.
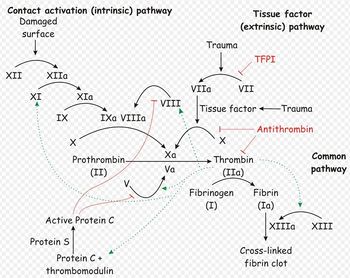
The platelet plug formed by primary haemostasis is fragile and must be reinforced in order to provide longer-term benefit. In secondary haemostasis, proteinaceous clotting factors interact in a cascade to produce fibrin to reinforce the clot. Two arms of the cascade are activated simultaneously to achieve coagulation: the intrinsic and extrinsic pathways. The intrinsic pathway is activated by contact with collagen due to vessel injury and involves the clotting factors XII, XI, IX and VIII. The extrinsic pathway is triggered by tissue injury and is effected via factor VII. These pathways progress independently before converging at the common pathway, which involves the factors X, V, II and I and ultimately results in the formation of fibrin from fibrinogen. Factors I, VII, IX and X are dependent upon vitamin K to become active.
The end product of haemostasis is a solid clot of fused platelets enclosed in a mesh of fibrin strands. It is important that uncontrolled, widespread clot formation is prevented, and so a fibrinolytic system exists to breakdown fibrin within blood clots. The two most important anticoagulants involved in fibrinolysis are antithrombin III (ATIII) and Protein C. The end products of fibinolysis are fibrin degratation products (FDPs).
Disorders of Haemostasis
Abnormalities can develop in any of the components of haemostasis. Disorders of primary haemostasis include vessel defects (i.e. vasculitis), thrombocytopenia (due to decreased production or increased destruction) and abnormalities in platelet function (e.g. congenital defects, disseminated intravascular coagulation). These lead to the occurence of multiple minor bleeds and prolonged bleeding. For example, petechial or ecchymotic haemorrhages may be seen on the skin and mucous membranes, or ocular bleeds may arise. Generally, intact secondary haemostasis prevents major haemorrhage in disorders of primary haemostasis. When secondary haemostasis is abnormal, larger bleeds are frequently seen. Haemothroax, haemoperitoneum, or haemoarthrosis may occur, in addition to subcutaneous and intramuscular haemorrhages. Petechiae and ecchymoses are not usually apparent, as intact primary haemostasis prevents minor capillary bleeding. Examples of secondary haemostatic disorders include clotting factor deficiencies (e.g. hepatic failure, vitamin K deficiency, hereditary disorders) and circulation of substances inhibitory to coagulation (FDPs in disseminated intravascular coagulation, lupus anticoagulant). If fibrinolysis is defective, thrombus formation and infarctions may result. Thrombus formation may be promoted by vascular damage, circulatory stasis or changes in anticoagulants or procoagulants. For example, ATIII may be decreased. This can occur by loss due to glomerular disease or accelarated consumption in disseminated intravascular coagulation or sepsis.
It is therefore important that all aspects of haemostasis can be evaluated. This will help to identify the phase affected and to pinpoint what the abnormality is. There are tests available to assess primary haemostasis, secondary haemostasis and fibrinolysis.
Tests Evaluating Primary Haemostasis
Primary haemostasis is dependent on the activity of platelets and, to a lesser extent, the blood vessels themselves. Platelets are the smallest solid formed component of blood, and are non-nucleated, flattened disc-shaped structures1. Their activity leads to vascoconstriction and the formation of platelet plugs to occlude vessel defects. Platelets develop in the bone marrow, and have a life span of around 7.5 days. Around two thirds of platelets are found in the circulation at any one time, with the remainder residing in the spleen1.
Apart from unusual cases involving vasculitis, there are two causes of defects in primary haemostasis: thrombocytopenia (reduced platelet number), or thrombocytopathia (defective platelet function)2.
Platelet Number
A platelet count can give valuable information in all critically ill animals and is an essential laboratory tests for patients with bleeding concerns. Platelet numbers may be rapidly estimated by examination of a stained blood smear, of quantified by manual or automated counting techniques. To estimate the platlet number using a blood smear, the slide should first be scanned for evidence of clumping that would artificially reduce the count. The average number of platelets in ten oil-immersion fields should be counted, and a mean calculated. Each platelet in a high-power field represents 15,000 platelets per microlitre2. A "normal" platelet count therefore gives aroung 10-15 platelets per oil-immersion field.
The reference range given for platelet number is usually around 200-500x109 per litre, although this varies depending on the laboratory used. Clinical signs due to thrombocytopenia are not commonly encountered until the platelet count drops below 50X109/l, when increased bleeding times may be seen. Haemorrhage during surgery becomes a concern with counts lower than 20X109/l, and spotaneous bleeding arises when platelets are fewer than 5X109/l2. These cut-offs are lowered if platelet function is concurrently affected, for example by the use of non-steroidal anti-inflammatory drugs1.
Buccal Mucosal Bleeding Time
The buccal mucosal bleeding time is a simple test that gives a rapid assessment of platelet function, providing platelet numbers are normal. If platelet numbers are below 50x109/l, this test should not be performed since the results will be affected by thrombocytopenia, making them unreliable. The small wound inflicted may also not stop bleeding easily.
To perform the BMBT test, a standardised tool producing a uniform incision is used2, 3 to make an incision in the buccal mucosa of the upper lip, and the time between making the incision and the cessation of bleeding is measured2 During the procedure the lip should be kept turned outwards, with excess blood being gently absorbed at a site away from the incision, without disturbing clot formation or applying pressure. Normally, bleeding should stop within 3 minutes, and a BMBT of greater than 5 minutes is considered prolonged2.
Acquired platelet function abnormalities are often drug induced, for example by non-steroidal anti-inflammatory drugs<sup.2, 3, or caused by uraemia. Hereditary defects in platelet function also exist, and von Willebrand's disease is the most common of these.
The BMBT is influenced by all the aspects of this phase, including vasoconstriction, platelet adherence and plately aggregation. Although this makes BMBT an effective screening test for the vascular/platelet (primary) phase of haemostasis, it also means it is not purely a test for thrombocytopathia, as it is often considered: BMBT depends on an intact vasospastic response and adequate platelet numbers as well as platelet function3. BMBT is also a fairly crude test, and has been found to be normal in some patients with a known platelet function disorder and vice versa3. Therefore, the results of this test should be interpreted with some caution.
Tests Evaluating Secondary Haemostasis
Secondary hemostasis describes the formation of a cross linked fibrin meshwork in the blood clot and is dependent on the soluble coagulation factors. Abnormalities in secondary coagulation can occur from insufficient coagulation factors or the presence of inactive coagulation factors. The soluble coagulation factors are traditionally divided into the intrinsic, extrinsic and common pathways, as described in the introduction.
Activated Clotting Time
The activated clotting time (ACT) allows rapid evaluation of secondary haemostasis. The ACT is the time taken for 2ml of fresh whole blood to clot in a tube with a contact activator (diatomaceous earth2), but an automated analyser can perform a test with a similar principle. The reaction must occur at body temperature to give a reliable indication of haemostatic ability: this can be achieved by the use of a warm water bath, or by holding the tubes under an arm. The normal ACT is 90-120 seconds and less than 75 seconds in dogs and cats respectively2.
The contact activator used in the ACT test triggers the intrinsic pathway, and so ACT allows assessment of the intrinsic and common pathways. ACT will therefore be prolonged when factors I, II, V, VIII, IX, X, XI or XII are deficient or abnormal, such as in DIC, liver disease, vitamin K antagonist toxicosis or haemophilia A or B2. Thrombocytopenia may also increase ACT.
Activated Partial Thromboplastin Time
The APTT is measures the time necessary to generate fibrin from activation of the intrinsic pathway3. It therefore assesses functionality of the components of the intrinsic and common pathways of coagulation. The test is performed on citrated plasma, and so blood should be collected into a sodium citrate tube if the APTT test is to be undertaken. Once a sample is obtained, factor XII is activated by an external agent that will not also activate factor VII, such as kaolin,1 3. Since the intrinsic arm of the cascade requires platelet factors to function, the test also provides a phospholipid emuslion in place of these factors. Calcium is added, the preparation is incubated, and the time for clumping of kaolin is measured. Classically, partial thromboplastin time was measured after activation by contact with a glass tube, but use of an external activating agent in the newer, "activated" partial thromboplastin time method makes results more reliable3.
APPT evaluates the same pathways as ACT, and so will be prolonged by abnormalities or deficiencies in factors XII, XI, IX, VIII, X, V, II or I. However, is not affected by thrombocytopenia and is also considered to be a more sensitive test than ACT: APTT becomes prolonged when 70% of a factor is depleted, compared to 90% depletion of ACT. APTT can also be prolonged in the presence of a circulating inhibitor to any of the intrinsic pathway factors. To differentiate factor deficiency from inhibition, a "mixing study" can be performed where the test is repeated on a 1:1 mix of patient and normal plasma. Complete correction indicates a deficiency, and partial or no resolution shows that an inhibitor is present. This difference stems from the above mentioned fact that the APTT will be normal in the presence of 50% normal activity3.
Conditions in which APTT is prolonged include inherited disorders, such as haemophilia A and B and other congential absences of intrinsic and common factors. Acquired factor deficiency also occurs, for example with vitamin K deficiency, liver dysfunction, prolonged bleeding or disseminated intravascular coagulation. The most common inhibitors found to prolong APTT are the antithrombins, which inhibit the activity of thrombin on the conversion of fibrinogen to fibrin. Examples include heparin and fibrin degradation products.
Occasionally, a shortened APTT is seen. This may reflect increased levels of activated factors in a hypercoagulable state, for example in the early stages of DIC3.
Prothrombin Time
Prothrombin time (PT) gives an assessment of the extrinsic and common pathways by measuring the time necessary to generate fibrin after activation of factor VII3. It is performed by an automated analyser2 using citrated plasma1, 3. Blood should therefore be collected into a sodium citrate tube if prothrombin time is to be performed. In basic terms, the test procedure involves adding thromoplastin to the patient's plasma, warming, adding calcium and recording the time taken to clot1.
A prolonged PT may reflect a factor deficiency or the presence of a circulating inhibitor of coagulation.Repeating the test using a mix of test plasma and "normal" plasma can help differentiate these possibilities: PT returns to normal limits when normal plasma is added to factor-deficient plasma, but no change is seen when this is added to plasma containing inibitors3. PT is more sensitive than APTT for factor deficiencies.
PT is affected by abnormalities or deficiencies in coagulation factors I, II, VII or X, for example in DIC, liver disease, or poisoning with vitamin K antagonists. Inherited defects are possible. PT is also prolonged by the presence of circulating anticoagulants. Inhibitors are often directed at factor X or thrombin and can include fibrin degradation products or heparin3.
Tests for Individual Clotting Factors
Some specialised laboratories offer tests for specific clotting factors. A sodium citrate sample is required
PIVKA
The vitamin K-dependent coagulation factors (FII, FVII, FIX, FX) are produced in the liver as nonfunctional precursors. These precursors are activated, in the presence of vitamin K, by carboxylation of their glutamic acid residues. This is achieved with the aid of an enzyme called gamma glutamyl carboxylase. Carboxylation of these amino acid residues allows these coagulation factors to bind calcium, which is essential for the adhesion of these factors to platelet phospholipid (which is necessary for the coagulation cascade to proceed). The inactive precursors are stored in the microsomal system of the liver and are absent in the circulation in normal animals. In the absence of vitamin K, there is an increase in these precursors, which spill into the circulation, as well as a depletion of the functional activated coagulation factors. The inactive precursors that accumulate in the circulation are called Proteins Induced by Vitamin K Antagonism or Absence.
The PIVKA test or thrombotest is a modification of the prothrombin time. The test uses diluted plasma (which creates longer clotting times than the PT) and a specific thrombotest reagent, which is reported to be most sensitive to deficiencies in factor X. Although claims have been made in the veterinary literature that this test is sensitive to both an increase in PIVKAs (which are supposed to inhibit the reaction) as well as a decrease in the functional coagulation factors II, VII, and X (especially factors VII and X), there is no proof that PIVKAs actually inhibit the reaction. Therefore, the PIVKA test should be considered as a modified PT that detects deficiencies in factors II, VII, and X. Because the clotting times are longer than the PT, the test may be more sensitive than the PT for abnormalities in these factors, however this was not supported by a recent study measuring PIVKAs, PT and APTT in dogs with various hemostatic disorders. Furthermore, the test is only offered by certain laboratories and the turnaround time is longer than the PT, therefore measurement of PIVKAs offers no advantage over the PT. In human patients, the nonfunctional noncarboxylated coagulation proteins are measured specifically using immunologic tests.
The PIVKA test is prolonged in vitamin K deficiency due to low vitamin K-dependent factor concentrations. This is usually due to vitamin K antagonism by anticoagulant rodenticides but can also be seen in cholestatic liver disorders, e.g. hepatic lipidosis in cats. Early studies suggested that the PIVKA test may be the first coagulation test to be prolonged after experimental administration of anticoagulant rodenticides to dogs, however no further studies have been done to support this claim. The PIVKA test, like the PT, is prolonged in some dogs with DIC and dogs with factor VII deficiency, indicating that it is not specific for vitamin K deficiency. Some authors believe that the PIVKA test is a useful guide as to which patients with liver disease and abnormal coagulation results will respond to vitamin K therapy (by normalization of the coagulation times). The PIVKA test is normal in dogs with inherited deficiencies of factors VIII, IX, and XI, and prekallikrein.
Tests Evaluating Fibrinolysis
Fibrin Degradation Products
Fibrin Degradation Products Definition
As a marker of fibrinolysis, fibrin degradation products (FDP), also known as fibrin split products (FSP), can be quantified by a test based on latex agglutination. The test uses antibodies to FSP which are measured using serial dilutions. Technique
Serum is prepared in a series of dilutions (e.g., ½, ¼, 1/16). Latex particles onto which have been absorbed antibodies to FSP are added. If agglutination is seen, the test is positive at that dilution. The test is reported as the most dilute sample that agglutinates. The normal value is less than ¼ to ⅙. Basic Science
Although well standardized and easy to perform, the FSP value may be difficult to interpret. The action of thrombin on fibrinogen is to cleave the protein and produce smaller compounds called fibrinopeptides, plus the fibrin monomer. This monomer polymerizes to form the fibrin gel. The gel is stabilized by the action of factor XIII, activated by thrombin.
The action of plasmin is to cleave both fibrinogen and fibrin. Its action is localized by its activation at the site of endothelial rupture, and the tight association of plasminogen and fibrin. The activity on fibrinogen forms small fragments, D and E. The action on fibrin polymer is to form larger fragments. These fragments are anticoagulants formed at the site of coagulation and serve to inhibit the action of thrombin on fibrinogen to form fibrin. Both are also measured by the technique described above. Clinical Significance
Increased FDP is the laboratory expression of increased fibrinolysis. This may be part of a local problem of fibrin generation such as brain trauma, chronic bleeding, vascular thrombosis, prostate surgery, uterine disorders, or malignancy, or a systemic process, usually DIC. Patients with severe liver disease can have increased fibrinolysis on the basis of poor clearance of circulating plasminogen activators.
References
- Fischbach, F T and Dunning, M B (2008) A manual of laboratory and diagnostic tests, Lippincott Williams and Wilkins.
- Hopper, K (2005) Interpreting coagulation tests. Proceedings of the 56th conference of La Società culturale italiana veterinari per animali da compagnia.
- Walker, K H et al (1990) Clinical Methods: The History, Physical and Laboratory Examinations (Third Edition), Butterworths.
- Howard, M R and (2008) Haematology: an illustrated colour text, Elsevier Health Sciences.