Dirofilaria immitis
| This article is still under construction. |
Description
Dirofilaria immitis is a nematode parasite that causes heartworm disease in dogs and, less commonly, cats and ferrets. Heartworm
disease is transmitted by mosquito bites and there are more than 70 species of mosquito that are able to transmit infection; Aedes, Anopheles and Culex are the most common vector species. Heartwoem disease has been reported in many countries with temperate and is particularly prevalent in the USA, Canada, and southern Europe. The introduction of the PETS travel scheme has increased the concern over Dirofilariasis in the UK.
Dirofilarias does have zoonotic potential: infected mosquitos can transmit Dirofilaria immitis to humans, but the infection does not become patent. The infective larvae instead reach the lungs, become encapsulated, and die causing granulomatous reactions called "coin lesions" in the process. These are only imporant because they may be confused with neoplastic metastasis to the lungs on radiographymerck.
Life Cycle
Dirofilaria immitis adults reach maturity and sexually reproduce in the pulmonary arteries and right ventricle. Adult males are around 15cm in length, and females are around 25cmmerck. After mating, female worms release larvae known as microfilariae (or L1) into the circulation. When a mosquito takes a blood meal from the infected dog or cat, microfilariae are ingested. Mosquitos are true intermediate hosts for Dirofilaria immitis, and microfilariae require a period of maturation to L2 and then L3 in the vector. The duration of this development depends upon environmental conditions. For example, maturation at 30°C takes around 8 days, but when temperatures are down to 18°C, this takes around one monthferasin. Below 14°C, development is halted and resumes when temperatures rise. In cooler climates, this means that transmission of heartworm disease to new canine or feline hosts can only occur in warmer months.
Once matured, L3 in the mosquito migrate to the labium of the vector, from which they erupt onto the host's skin as the mosquito feeds. Larvae then migrate into the bite wound and as most dogs are highly susceptible to heartworm disease, most L3 do establish infection. It takes 2-3 days for L3 to moult to L4, which remain in the subcutaneous tissues for up to two months before becoming young adults (L5) and migrating to the pulmonary arteries.
Cats differ from dogs in that they are more resistant to infection with Dirofilaria immitis. A lower percentage of exposed cats develop adult infections, and when this does occur the burden is usually lowmerck. L5 in the pulmonary arteries also have a relatively short (2 year) survival time in cats.
Pathogenesis
Heartworm disease is primarily a cardiopulmonary disease. the severity and extent of lesion depends on the number and location of adult worms duration of infection, and host activity level.. The presence of parasites in the pulomnary arteries causes mechanical irritation and proliferation of the intima, redulting in narrowing and occlusion of the vessels, which leads to pulmonary hypertension. perivascular cuffing with inflammatory cells, including infiltration of high numbers of eosinophils. Severe pulmonary arterial disease may cause increased permability of the lung vessls with periarterial oedema, and intersitial and alveolar cellular infiltrate, which can result in irreversible lung fibrosis. Pulmonary thromboembolism, due to platelet aggregation induces by the parasis or in response to the deat (spontaneous or induced by adultidicat treatment) of adult worms is another possible sequela of heartworm disease. chronic lesions and subsequent scarring
HW-associated inflammatory mediators that induce immune responses in the lungs and kidneys (immune complex glomerulonephritis) cause vasoconstriction and possibly bronchoconstriction. Leakage of plasma and inflammatory mediators from small vessels and capillaries causes parenchymal lung inflammation and edema. Pulmonary arterial constriction causes increased flow velocity, especially with exertion, and resultant shear stresses further damage the endothelium. The process of endothelial damage, vasoconstriction, increased flow velocity, and local ischemia is a vicious cycle. Inflammation with ischemia can result in irreversible interstitial fibrosis.
In some severe cases, worms can migreat to the right ventricle, right artrium and caudal vena cava. This retrografe migration induces incompetence of the tricuspid valve which, in association with concurrent pulmonary hypertension, is responsible for the clinical signs of right-sides heart failure (e.g. jugular distension, liver congestion, ascites). In addition, red blood cell memranes may rupture as the vells flow through the mass of parasits, causing haemolysis and haemoglobinaemia. The concomitant presence of acture right-sided heart failure and intravascular haemolysis is referred to as caval syndrome. Severe cases of caval syndrome can also be characterised by the present of adult worms in the caudal vena cava, thromboembolic events and disseminated intravascular coagulation. Caval syndrom is less common in cats due to the lighter woem burden.
In cats, hertwoms disease generally induces a diffuse plumonary infiltrate and signs of eosinophilis pneumonia. The death od adult worms may cause acute pulmonary embolism with severe haemorrhage and oedema of the affected lobe. Occasionally, immature nematodes cna migrate to sites other than the heart and plumonary areties, causing ectopic infection. Localisation of D. immitis has been repored in the eye, CNS, systemic arteries and subcutaneous tissues. Ectopic infections are more commonly seen in cats than in dogs, suggesting that the parasite is not well adapted to feline hosts.
Signalment
The risk of Dirofilaria immitis infection is greatest in outdoor dogs and cats. Dogs of any age may be affected, but infections are most common in 3 to 8 year old dogs, and medium and large breeds are over-representedmerck, fmc. In cats, there are no breed or age predispositions, but males are more frequently affectedfmc. There are no age predilections in ferretsmerck.
Diagnosis
Diagnosis:
- Physical examination:
- signs of heart disease
- lung involvement
- Radiography:
- enlargement of right heart, main pulmonary arteries; arteries in lung lobes with thickening and tortuosity; inflammation in surrounding tissues
- ECG:
- right axis deviation → deep S waves
- Echocardiography:
- if post caval syndrome suspected - right ventricular enlargement with worms in ventricle appearing as parallel lines.
Clinical pathology:
- needed alongside physical examination and other tests to determine treatment strategy and prognosis.
Parasite detection:
- methods for demonstrating microfilariae in blood:
- wet blood smear (okay for quick look, but insensitive) = D. immitis not progressively motile
- Knott's test = red blood cells lysed; stained sediment examined
- micropore filter = blood forced through; microfilariae held on filter; stained and examined
- antibody detection ELISA = not reliable in dogs, but it is the best for cats (although some false positives)
- antigen detection ELISA (using specific antigen from adult female worm) = reliable positives from 5-7months post-infection in dogs; although occasional false negatives occur → not useful for cats
- the immunochromatographic test (ICT) uses coloured gold colloidal particles tagged to monoclonal antibodies to visualise the presence of adult worm antigen - performance similar to antigen detection ELISA, but quicker and easier to do (but not as quantitative as some ELISAs are)
- operator error can give false positives, therefore best to confirm result with another test.
Clinical Signs
In dogs, infection should be identified by serologic testing prior to the onset of clinical signs; however, it should be kept in mind that HW antigenemia and microfilaremia do not appear until ~5 and 6.5 mo postinfection, respectively. When dogs are not administered a preventative and are not appropriately tested, clinical signs such as coughing, exercise intolerance, unthriftiness, dyspnea, cyanosis, hemoptysis, syncope, epistaxis, and ascites (right-sided CHF) are likely to develop. The frequency and severity of clinical signs correlate to lung pathology and level of patient activity. Signs are often not observed in sedentary dogs, even though the worm burden may be relatively high. Infected dogs experiencing a dramatic increase in activity, such as during hunting seasons, may develop overt clinical signs. Canine HW disease can be classified by physical examination, thoracic radiographs, urinalysis, and PCV. Class I is asymptomatic to mild HW disease, with no clinical or radiographic signs and no laboratory abnormalities. Subjective signs such as loss of condition, decreased exercise tolerance, or occasional cough might be seen. Class II is moderate HW disease, characterized by an occasional cough and mild-to-moderate exercise intolerance. A slight loss of condition, increased lung sounds, and mild to moderate radiographic changes, such as right ventricular enlargement, are present. Laboratory results may show anemia and proteinuria. Class III is severe disease variably characterized by anemia, weight loss, exercise intolerance, tachypnea at rest, severe or persistent coughing, dyspnea, hemoptysis, syncope, and ascites. Severely abnormal radiographs may show right ventricular hypertrophy, enlargement of the main pulmonary artery, and diffuse pulmonary densities. Laboratory results indicate marked anemia, thrombocytopenia, and proteinuria. Electrocardiographic evidence of right ventricular hypertrophy is often present. Class IV, also known as the caval syndrome, is characterized by sudden onset with collapse, hemoglobinuria, and respiratory distress. If surgery is not immediately instituted, this syndrome is usually fatal. nfected cats may be asymptomatic or exhibit intermittent coughing, dyspnea, vomiting, lethargy, anorexia, or weight loss. The symptoms often resemble those of feline asthma. In general, signs are most prevalent during periods when worms die, including when young adult worms arrive in the lungs. Antigen tests in cats are negative during the early eosinophilic pneumonitis syndrome, although antibody tests may be positive. Subsequently, clinical signs often resolve and may not reappear for months. Cats harboring mature worms may exhibit intermittent vomiting, lethargy, coughing, or episodic dyspnea. HW death can lead to acute respiratory distress and shock, which may be fatal and appears to be the consequence of pulmonary thrombosis.
Diagnostic Imaging
In dogs, echocardiography is relatively unimportant as a diagnostic tool. Worms observed in the right heart and vena cava are associated with high-burden infection with or without caval syndrome. Severe, chronic pulmonary hypertension causes right ventricular hypertrophy, septal flattening, underloading of the left heart, and high-velocity tricuspid and pulmonic regurgitation. The ECG of infected dogs is usually normal. Right ventricular hypertrophy patterns are seen when there is severe, chronic pulmonary hypertension and are associated with overt or impending right-sided CHF (ascites). Heart rhythm disturbances are usually absent or mild, but atrial fibrillation is an occasional complication in dogs with Class III disease.
In cats, worms can usually be imaged on echocardiography. Parallel hyperechoic lines, which are an image from the heartworm cuticle, may be seen in the right heart and pulmonary arteries. High worm burdens may be associated with worms in the right heart. Echocardiography is more important in cats than dogs because of the increased difficulty of diagnosis and the high sensitivity of the test in experienced hands.
n dogs, thoracic radiography provides the most information on disease severity and is a good screening tool for dogs with clinical signs compatible with dirofilariasis. Class III infections are characterized by a large main pulmonary artery segment and dilated, tortuous caudal lobar pulmonary arteries. If the latter are ≥1.5 times the diameter of the 9th rib at their point of superimposition, then severe pathology is present. Right ventricular enlargement may also be seen. Fluffy, ill-defined parenchymal infiltrates of variable extent often surround the caudal lobar arteries, usually worst in the right caudal lobe, in advanced disease. The infiltrate may improve with cage confinement with or without anti-inflammatory dosages of a corticosteroid. In cats, cardiac changes are less common. The caudal lobar arteries normally appear relatively large, but are larger still with heartworm infection. Patchy parenchymal infiltrates may also be present in cats with respiratory signs. The main pulmonary artery segment usually is not visible due to its relatively midline location.
Laboratory Tests
In addition to special diagnostic tests in both cats and dogs, a CBC, chemistry profile, urinalysis, and particularly thoracic radiographs are indicated. Laboratory data are often normal. Eosinophilia and basophilia are common and together suggest occult dirofilariasis or allergic lung disease. Eosinophilia surges as the L5 arrive in the pulmonary arteries. Subsequently, eosinophil counts vary but are usually high in dogs with immune-mediated occult infections, especially if eosinophilic pneumonitis develops (<10% of total infections). Hyperglobulinemia may be present in dogs and cats due to antigenic stimulation. Hypoalbuminemia in dogs is associated with severe immune-complex glomerulonephritis or right-sided CHF. Serum ALT and alkaline phosphatase are occasionally increased, but do not correlate well with abnormal liver function, efficacy of adulticide treatment, or risk of drug toxicity. Urinalysis may reveal proteinuria that can be semiquantitated by a urine protein:creatinine ratio. Occasionally, severe glomerulonephritis or amyloidosis can lead to hypoalbuminemia and nephrotic syndrome. Dogs with hypoalbuminemia secondary to glomerular disease also lose antithrombin III and are at risk for thromboembolic disease. Hemoglobinuria is associated with Class III disease when RBC are lysed in the pulmonary circulation by fibrin deposition. Heparin therapy (75-100 U/kg, SC, tid) is indicated. Hemoglobinuria is also a classic sign of the vena caval syndrome.
he antigen detection test is the preferred diagnostic method for asymptomatic dogs or when seeking verification of a suspected HW infection. This is the most sensitive diagnostic method available to veterinary practitioners. Even in areas where the prevalence of HW infection is high, ~20% of infected dogs may not be microfilaremic. Also, monthly macrolide prophylaxis induces embryo stasis in female dirofilariae. Available antigen detection tests are very sensitive and specific. To determine when testing might become useful, it is advisable to add a predetection period to the approximate date on which infection may have been possible. A reasonable interval is 7 mo. There is generally no need to test a dog for antigen or microfilariae prior to ~7 mo of age. The level of antigenemia is directly related to the number of mature female worms present. At least 90% of dogs harboring ≥3 adult females will test positive. In general, strong-quick positive reactions correlate with relatively high worm burdens. For low-burden suspects, commercial laboratory-based microwell titer tests are the most sensitive.
Pathology
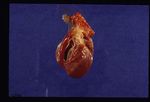
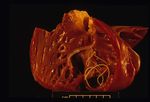
On post-mortem examination, the right side of the heart is found to be enlarged. There is proliferation of the pulmonary arterial myointima, as well as pulmonary thromboembolism and haemorrhage. If right-sided congestive heart failure was present in life, the hepatomegaly and hepatic congestion will be seen.
Treatment
Treatment in Dogs: The extent of the preadulticide evaluation will vary depending on the clinical status of the patient and the likelihood of coexisting diseases that may affect the outcome of treatment. Clinical laboratory data should be collected selectively to complement information obtained from a thorough history, physical examination, antigen test, and usually thoracic radiography. The most important variables influencing the probability of postadulticide thromboembolic complications and the outcome of treatment are the extent of concurrent pulmonary vascular disease and the severity of infection. Assessment of cardiopulmonary status is indispensable for evaluating a patient’s prognosis. Postadulticide pulmonary thromboembolic complications are most likely to occur in heavily infected dogs already exhibiting clinical and radiographic signs of severe pulmonary arterial vascular obstruction, especially if CHF is present. The only available heartworm adulticide is melarsomine dihydrochloride, which is effective against mature (adult) and immature heartworms of both genders. For Class I and II patients, melarsomine is given at 2.5 mg/kg, deep IM in the epaxial (lumbar) musculature in the L3-L5 region using a 22 g needle (1 in. long for dogs <10 kg or 1.5 in. for dogs >10 kg). Pressure is applied during delivery and for 1 min after the needle is withdrawn to prevent SC leakage. The procedure is repeated on the opposite side 24 hr later. Approximately one-third of dogs will exhibit local pain, swelling, soreness with movement, or sterile abscessation at the injection site. Local fibrosis is uncommon. Dogs with high worm burdens are at risk of severe pulmonary thromboembolism from several days to 6 wk postadulticide. Dogs with Class III infection receive the alternate (split-dose) regimen of 1 injection, followed in 1 mo by 2 injections 24 hr apart. Administration of a single initial dose results in a graded (~50%) worm kill and reduced pulmonary complications. By initially killing few worms and completing the treatment in 2 stages, the cumulative impact of worm emboli on severely diseased pulmonary arteries and lungs can be reduced. This 3-injection protocol is becoming the treatment of choice of many veterinarians regardless of stage of disease, due to its increased safety and efficacy. Other treatment protocols recommend the administration of prophylactic doses of ivermectin for 1-6 mo prior to administration of melarsomine, if the clinical presentation does not demand immediate intervention. The rationale for this approach is to greatly reduce or eliminate circulating microfilariae and migrating D immitis larvae, stunt immature HW, and reduce female worm mass by destroying the reproductive system. This results in reduced antigenic mass, which in turns reduces the risk of pulmonary thromboembolism. Following melarsomine injection, exercise must be severely restricted for 4-6 wk to minimize thromboembolic lung complications. A low cardiac output should be maintained in order to reduce thrombosis and endothelial damage and facilitate lung repair. Adverse effects of melarsomine are otherwise limited to local inflammation, brief low-grade fever, and salivation. Hepatic and renal toxicity are seldom seen. Class III patients should be stabilized prior to melarsomine administration. Stabilizing treatment variably includes cage confinement, oxygen, corticosteroids, and heparin (75-100 U/kg, SC, tid) for 1 wk prior to the alternate melarsomine treatment protocol. Patients with right-sided CHF should be treated with furosemide (1-2 mg/kg, bid), a low-dose angiotensin-converting enzyme (ACE) inhibitor such as enalapril (0.25 mg/kg, bid, possibly increased to 0.5 mg/kg, bid after 1 wk pending renal function test results), and a restricted sodium diet. Digoxin, digitoxin, and arteriolar dilators, such as hydralazine and amlodipine, should not be administered. Digoxin is not effective for cor pulmonale; arteriolar dilators, and occasionally even ACE inhibitors, are likely to cause systemic hypotension. Postadulticide thromboembolic complications can occur 2-30 days following treatment, with signs most likely 14-21 days after treatment. Clinical signs are coughing, hemoptysis, dyspnea, tachypnea, lethargy, anorexia, and fever. Laboratory findings may include an inflammatory leukogram, thrombocytopenia, and prolonged activated clotting time or prothrombin time. A postinjection increase in serum CK may be noted. Local or disseminated intravascular coagulopathy may occur when platelet counts are <100,000/µL. Treatment for severe thromboembolism should include oxygen, cage confinement, a corticosteroid at an anti-inflammatory dosage (eg, prednisone at 1.0 mg/kg, PO, sid), and low-dose heparin (75-100 U/kg, SC, tid) for several days to 1 wk. Most dogs respond within 24 hr. Severe lung injury is likely if, after 24 hr of oxygen therapy, no improvement is noted and partial pressures of oxygen remain <70 mm Hg. Both the standard melarsomine protocol and the alternate regimen kill all or most worms in ~75% of dogs. Antigen testing is performed 6 mo after the first 2 doses of the standard protocol or 4-6 mo after the third dose of the alternate protocol. A positive test result should be followed by retreatment (2 injections, 24 hr apart) if the antigen test is strongly positive, if the patient is still symptomatic, and if the patient is an athlete or a working dog. Mild infection, a weakly positive antigen test, absence of clinical signs, advanced age, and a sedentary dog are factors that may negate the need for a repeat melarsomine treatment. Maintaining dogs on ivermectin/pyrantel pamoate to slowly kill residual worms over the following 20 mo is an alternative in nonperforming dogs with a post-melarsomine weakly positive antigen test result. Ivermectin/pyrantel pamoate administered monthly for ~2 yr beginning at 5-7 mo post-L3 inoculation eradicates most adult worms. Further, during this time period, some older worms are also killed. However, the use of ivermectin/pyrantel pamoate is seldom a substitute for melarsomine treatment because the slow kill may allow pulmonary pathology to progress in the interim. In caval syndrome cases (class IV), surgical removal of worms from the right atrium and orifice of the tricuspid valve is necessary to save the life of the dog. This may be accomplished by using local anesthesia and either a rigid or flexible alligator forceps, or an intravascular retrieval snare, introduced preferentially via the right external jugular vein. With fluoroscopic guidance, if available, the instrument should continue to be passed until worms can no longer be retrieved. Immediately following a successful operation, the clinical signs should lessen or disappear. Fluid therapy may be necessary in critically ill, hypovolemic dogs to restore hemodynamic and renal function. Within a few weeks following recovery from surgery, adulticide chemotherapy is recommended to eliminate any remaining worms, particularly if many are still visible echocardiographically. Microfilaricide Treatment: At specific preventive dosages, the macrolide preventative drugs are effective microfilaricides, although not approved by the FDA for this purpose. Adverse reactions may occur in dogs with high microfilarial counts (>40,000/µL), depending on the type of macrolide given. However, the microfilarial count is usually lower, and mild adverse reactions occur in ~10% of dogs. Most adverse reactions are limited to brief salivation and defecation, occurring within hours and lasting up to several hours. Dogs, especially small dogs (<10 kg), with high microfilarial counts (>40,000/µL) may develop tachycardia, tachypnea, pale mucous membranes, lethargy, retching, diarrhea, and even shock. Treatment includes IV balanced electrolyte solution and a soluble corticosteroid. Recovery is usually rapid when treatment is administered quickly. Microfilarial counts are not routinely performed, and thus severe reactions are seldom expected. Treatment specifically targeting circulating microfilariae may be started as early as 3-4 wk following adulticide administration. More commonly, microfilariae are eventually eliminated, even from non-adulticide-treated dogs, after several months of treatment with prophylactic doses of the macrocyclic lactones. No drugs are currently approved as microfilaricides by the FDA. However, licensed veterinarians are permitted extra-label use of certain drugs if a valid veterinarian-client-patient relationship exists. The use of monthly administered HW chemoprophylactics as microfilaricides is governed by this regulation. The macrocyclic lactones are the safest and most effective microfilaricidal drugs available. Livestock preparations of these drugs should not be used to achieve higher doses for the purpose of obtaining more rapid results. The macrolide of choice for killing microfilariae quickly is milbemycin (0.5 mg/kg, PO, 1 dose). Performance of a microfilariae test is recommended at the time the antigen test is performed (6 mo after the adulticide treatment). Developing Larvae: Ivermectin/pyrantel pamoate, administered monthly for 1 yr to dogs with larvae that are no more than 4 mo post-L3 inoculation, prevents the development of infection. Continuous monthly administration of prophylactic doses of ivermectin, alone or in combination with pyrantel pamoate, is also highly effective against late precardiac larvae and young (<7 mo postinfection) quasi-adult HW. Comparable capability of the other macrocylic lactones has not been reported. This extended protection is important in dogs of unknown medical history that may have acquired HW infections because of lack of preventive drug administration or lack of compliance. Back to top Treatment in Cats: There is currently no satisfactory treatment approach to heartworm infections in cats. Infection often is lethal, and a safe and effective melarsomine protocol has not yet been developed. Thus, all cats in canine HW-endemic regions should receive drug prophylaxis. The adult heartworm lifespan in cats is probably ≤2 yr, so spontaneous recovery is possible. Cats may remain asymptomatic, experience episodic vomiting and/or episodic dyspnea (resembling asthma), may die suddenly from pulmonary thromboembolism, or rarely, develop CHF. With each worm death, pulmonary complications occur. There does not appear to be an association between the presence, absence, or severity of clinical signs and the likelihood of acute complications. Many cats are managed conservatively with restricted activity and corticosteroid therapy, such as prednisolone (1.0-2.0 mg/kg, PO, every 24-48 hr). Steroids reduce the severity of vomiting and respiratory signs. The hope is that episodes of pulmonary complications will not prove fatal as the worms die. Barring superinfection, 25-50% of cats may survive with this approach. Serial antibody testing (at 6-mo intervals) can be used to monitor status. Surgical retrieval of worms from the right atrium, right ventricle, and vena cavae via jugular venotomy can be attempted in patients with high worm burdens detected by echocardiography. An endoscopic basket or horsehair brush can be advanced via the right jugular vein under fluoroscopy. Back to top
Prevention
Heartworm infection is completely preventable with macrolide prophylaxis. Preventive therapy in dogs is recommended beginning at 6-8 wk of age. No testing is necessary at this age. When started at ~1 yr of age, an antigen test is recommended. Before starting a prophylactic regime, all mature dogs that may have been infected >7 mo earlier should be antigen tested and, in appropriate instances, tested for presence of microfilariae. The determination of HW status before starting chemoprophylaxis will avoid unnecessary delay in detecting subclinical infections, and potential confusion concerning effectiveness of the preventive program, if a pre-existing infection becomes evident after beginning chemoprophylaxis. Year-round prevention is advised, but in northern climates, chemoprophylaxis is often initiated in the spring and continued through November. Year-round macrolide administration will arrest the development of larval stages (L3 and L4) that might have occurred prior to preventative initiation or when monthly doses were missed. The macrolide preventives ivermectin, milbemycin oxime, moxidectin, and selamectin are safe and effective as prescribed for all breeds. Ivermectin/pyrantel pamoate (hookworms and roundworms) and milbemycin (hookworms, roundworms, and whipworms) also provide control of intestinal nematodes. Milbemycin, however, kills microfilariae (L1) quickly and shock can occur in the face of high microfilarial concentrations. Thus, milbemycin is not administered as a preventive in dogs with microfilariae. Selamectin is administered topically at a monthly dosage of ~6 mg/kg and also kills adult fleas and prevents flea eggs from hatching for 1 mo. It also is indicated for the treatment and control of Otodectes cynotis in dogs and cats, sarcoptic mange, Dermacentor variabilis infestations in dogs, Ancylostoma tubaeforme , and Toxocara cati in cats. Annual antigen testing is recommended because overall owner compliance with macrolide prophylaxis is only ~50%. The injectable form of moxidectin is effective for at least 6 mo following 1 injection but use in microfilaremic dogs is not advised. At the time of publication, this formulation was not available in the USA because of concerns regarding toxicity in dogs. With macrolide administration for 6 mo and longer, microfilariae production by female worms ceases and antigen testing is required for detection of infection. Heartworm prevention is recommended for all cats in endemic regions, regardless of housing status, because of the potential severe consequences. Ivermectin for cats is safe and effective at 25 µg/kg, PO, once monthly. At this dose, the formulation is also effective against Ancylostoma tubaeforme and A braziliense . Preventive treatment should be initiated in all adult cats, in kittens 6 wk of age, and continued lifelong. Annual antigen and antibody testing is of limited value in cats receiving prophylaxis.
Prognosis
Links
References
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition), Merial.
- Ridyard, A (2005) Heartworm and lungworm in dogs and cats in the UK, In Practice, 27(3), 147-153.
- Ferasin, L (2004) Disease risks for the travelling pet: Heartworm disease, In Practice, 26(6), 350-357.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in cats. Dirofilaria immitis and D. repens in dog and cat and human infections, 126-132.
- Venco, L (2007) Heartworm (Dirofilaria immitis) disease in dogs. Dirofilaria immitis and D. repens in dog and cat and human infections, 117-125.