Description
Tuberculosis in cattle is caused by Mycobacterium bovis. It is a chronic disease characterised by nodular lesions in any organ, although the respiratory system is most commonly affected. The nodules often become nectroic with a caseous centre. The primary lesions may disseminate to involve other body systems.
A higher level of infection is required to establish the alimentary form of the disease and this is reflected by its lower incidence in comparison to the respiratory form.
Inhalation of ruminal gases is the most common route of entry for the mycobacterium organism, and spread of the disease is usually via cow to cow contant. Cattle can also become infected by ingestion of the causative agent, this is thought to be the route of entry when the badger is involved, by infecting grazing land or water troughs. Calves with infected dams can become affected via the milk, and intrauterine infection at coitus has been reported.
Historically bovine TB was a major cuase of human TB, but the introduction of tuberculin testing and slaughter, meat inspection at abatoirs, the pasturisation of milk and the BCG vaccination has dramatically reduced transmission to humans and bovine TB as a cuase of human disease is now very low indeed.
The disease is of serious economic importance to farmers becasue of the stringent control measures which remain in place. These include the slaughter of infected animals and movement restrictions placed on farms with reactors or inconclusive results.
Signalment
The disease usually affects heifers or young stock but cases can occur in cattle of any age. TB is more common in dairy herds.
Incidence of the disease has increased over the past 15 years; it is prevelent in Wales and the south west of England but is re-emerging in other parts of the UK such as the west Midlands and north-west England.
Most warm blooded animals are susceptible to bovine TB and can act as a resevoir for infection. The disease in cattle has been associated with wildlife species in a number of countries; the European badger and red deer in the UK, opossums and ferrets in New zealand, mule deer, white-tailed deer, elk, and bison in North America and water buffalo in Australia.
Diagnosis
The intradermal comparative tuberculin test is widely used in the UK for diagnosis of the disease. Two injections are give subcutaneously in the neck of cattle, one is avian and the second bovine tuberculin purified protein derivative (PPD). The thickness of the skin is recorded at each injection site. The test is read 72 hours later, and the thickness of the skin is remeasured. Interpretation is based on finding a swelling or increase in skin thinkness at the site of the injection. A comparison must be made between the reaction to avian and the bovine tuberculin to account for cross reactivity with related diseases, such as atypical mycobacteriosis, or paratuberculosis- Johne's disease.
A single intradermal test is used in many countries but has the disadvantage of giving reactors to avian tuberculosis and Johne's disease.
Clinical Signs
Location and severity depend on which body systems are affected and the advancement of the disease. Due to the tesing and slaughter policy most cases in the UK are idenified before development of clinical signs.
Respiratory form
- Chronic cough- soft and productive
- Tachypnoea
- Dyspnoea
- Dull areas on auscultation of the lungs in advanced cases
Alimentary form
- Few clinical signs
- Diarrhoea
- Bloat
Mammary involvement is now rarely seen, the uterine form is also uncommon but may reult in abortion.
Laboratory Tests
An ELISA test has been developed but is not widely used. The gamma interferon test can also be used for diagnosis of the condtion.
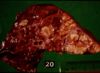
Pathology
Treatment
Treatment is not usually an option due to the chronic nature of the disease, zoonotic potential and test and slaughter policy.
Control in many countries is centered on tuberculin testing with slaughter of reactors and movement restrictions to the premises. Research work continues into the use of vacination, or a cull strategy for the associated wildlife populations.
Prognosis
Poor.
References
- Andrews, A.H, Blowey, R.W, Boyd, H and Eddy, R.G. (2004) Bovine Medicine (Second edition), Blackwell Publishing
- Reside primarily within macrophages where they multiply and result in characteristic granulomatous inflammation (macrophages and giant cells, epithelioid cells)
- Cattle can be infected by inhalation of the organism or through milk
- The primary complex
- Describes the initial focus of infection at the portal of entry (lungs) plus involvement of regional lymph nodes
- 90% of cases exhibit the pulmonary form
- Grossly:
- Small tubercles in dorsocaudal subpleural areas which progress to larger confluent areas of caseous necrosis
- Usually start at bronchio-alveolar junction an progress to the alveoli
- Caseous lesions, may calcify or be encapsulated
- Multiple foci may coalesce
- Ulcers in trachea and bronchi due to coughed up bacteria
- Spreads into pleura
- Microscopically:
- Typical granulomatous inflammation
- Epitheliod and giant cells at centre of tubercles
- Macrophages with ingested bacteria, forming epithelioid cells - large vesicular nuclei, abundant pale cytoplasm
- Giant cells, formed by fusion of macrophages, with multiple nuclei
- Narrow layer of lymphocytes, mononuclear cells and plasma cells at the periphery of the tubercle
- With time, peripheral fibroplasia and central necrosis develop
- If the infection is not contained in the primary complex described above, the mycobacteria can disseminate via lymphatics to other organs and lymph nodes
- This can allow the development of miliary tuberculosis, i.e. numerous small foci of infection in many organs/ tissues
- inhalation of Mycobacterium bovis most common via droplets
- some tubercle bacilli enter the lymph and travel to the bronchial or mediastinal nodes
- inhaled bacilli reach the alveoli, set up a focus of inflammation
- phagocytosed by alveolar macrophages
- two processes may develop if the animal has not encountered the organism before:
- - the organism may grow in the phagocytes as intracellular parasites
- - produces a nodule of parasitised swollen macrophages known as a tuburculous nodule or a tubercle granuloma
- - ultimately, macrophages are killed and infection spreads
- - the organism may be broken down and some antigens taken up by the immune system
- - cell mediated immune system produces cytotoxic T-lymphocytes
- - T-lymphocytes can attack and destroy cells harbouring bacilli
- - leads to type IV (delayedd type) hypesensitivity
- - 'caseous' or cheesy type of necrosis
- - if bacterium destroyed, further infection/disease is prevented
Tuberculosis pleurisy
caseous lymph node ruptures results from extensive tissue necrosis if located in lung alveoli, the follicle may rupture into a bronchus, causing spread of the disease to all the other lobules served by that bronchus the necrosis may erode the wall of a large pulmonary vessel resulting in fatal haemoptysis.