Rift Valley Fever
Also Known As – RVF
Caused By – Rift Valley Fever Virus - RVFV
Introduction
Rift Valley Fever is a viral disease caused by a bunyavirus.
It infects cattle, sheep, goats, camels and people.
RVF causes significant economic losses in Africa, both directly through its clinical cases and also as an obstruction to the improvement of breeding stock due to the susceptibility of most imported livestock breeds into endemic areas.
This disease is notifiable to the World Organisation for Animal Health (OIE)
Distribution
Africa
Huge epizootics develop, affecting up to 90% of a group, usually in 5-15 year cycles.
RVF is transmitted by a wide range of mosquitoes including Anopheles spp. and Culex spp. and also Hyalomma spp. ticks and the stable fly. Some trans-ovarial transmission is also thought to occur. Climate and weather play a huge role in the emergence and survival of these vectors and thus also in amplification of the virus. The mosquitoes require cloud cover and regular, significant precipitation.
The disease follows the rainy season in most countries.
Signalment
Cattle, sheep, goats and humans are important hosts. This is due in part to their presence in huge numbers in epizootic areas and therefore ability to greatly amplify viral presence in a population and transmit to others and humans.
Bos Taurus cattle and other European breed imported into Africa appear highly susceptible to RVF.
Indigenous breeds appear to be resistant to disease, as do pigs.
Cats, dogs, rats and other rodents seem to be accidental hosts infected by mosquitoes.
Humans working closely with animals or ingesting raw animal products, e.g. in rituals, are most predisposed.
Clinical Signs
Abortions occurring in huge storms with high mortality in both neonates and adults are characteristic of disease. Agalactia may also develop.
Vomiting and diarrhoeamay involve melaena and haematochezia.
Tachycardia, cyanosis, petechiation, haemorrhage and clotting defects are haematological consequences of RVF.
The respiratory disease of RVF is non-specific: Purulent nasal discharge, epistaxis, tachypnoea and dyspnoea.
Fever, lymphadenopathy, depression and lethargy usually accompany infection.
Hepatitis may cause consequent photosensitisation.
In young animals, peracute disease causes anorexia, listlessness, collapse and death.
Humans develop malarial-like disease. High risk individuals include farmers, veterinarians and abattoir staff. Mild disease is most common but severe hepatitis, encephalitis and ocular damage can develop. The usual presentation is of sudden onset fever, myalgia, biphasic behaviour and gastrointestinal disease.
Diagnosis
Sudden onset of acute debilitating disease in man and abortion/neonatal death in domestic animals should raise suspicion in appropriate countries.
Viral isolation can be performed from placenta, foetal liver and other tissues. The virus can also be innoculated into tissue cultures and diagnosed by Fluorescent Antibody Testing (FAT) or immune-peroxidase staining.
Fixed liver samples can be immunostained,and sera from aborted animals examined to confirm viral presence and are both simple and sensitive.
IgM ELISA can also be performed on serum.
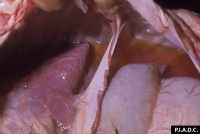
On necropsy, in the viraemic stage, widespread petechiae and ecchymoses on serous surfaces and organs will be seen and extravasated blood present in the body cavities.
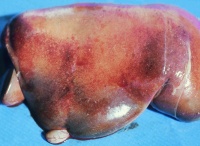
In older animals, the liver is enlarged and inflamed, with many foci of necrosis which are bronzed and jaundiced. The gall bladder may also be distended and haemorrhagic. Lymph nodes are enlarged and their germinal centres may be necrotic on closer examination. Extensive subcapsular haemorrhage in the spleen is usual. Renal changes include oedema and congestion. Epicardial and endocardial haemorrhages are often present on the heart.
Treatment
No treatment is available.
Control
Modified live and inactivated vaccines are available. Live vaccination is only recommended in non-pregnant animals due to its ability to cause abortion and neurological deficits in lambs. In epizootic situations though, this risk may well be worth taking.
Inactivated vaccines are ineffective during epizootics and therefore less widely used than modified live strains.
Mosquito and larval control is extremely valuable. Slow release larvicides such as methoprene can be applied to well defined mosquito breeding areas.
Sentinel cattle are used for epidemiological surveillance, and are tested 2-3months after the seasonal rains.
| Rift Valley Fever Learning Resources | |
|---|---|
 Test your knowledge using flashcard type questions |
Rift Valley Fever Flashcards |
 Search for recent publications via CAB Abstract (CABI log in required) |
Rift Valley Fever Publications |
References
Animal Health & ProductIon Compendium, Rift Valley Fever datasheet, accessed 08/06/2011 @ http://www.cabi.org/ahpc/
Animal Health & ProductIon Compendium, Rift Valley Fever Virus datasheet, accessed 08/06/2011 @ http://www.cabi.org/ahpc/