CNS Response to Injury - Pathology
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
|
|
Introduction
- The CNS is composed of two major cell types:
- Neurons
- Glial cells, which include:
- Astrocytes
- Oligodendrocytes
- Microglial cells
- Ependymal cells
- Choroid plexus epithelial cells
- The response to injury varies with the cell type injured.
Response of Neurons to Injury
- Neurons are particularly vulnerable to injury, due to their:
- High metabolic rate
- Small capacity to store energy
- Lack of regenerative ability
- Axons being very dependent on the cell body.
- Axons cannot make their own protein as they have no Nissl substance.
- The cell body produces the axon's protein and disposes of its waste.
- Death or damage of the cell body causes axon degeneration.
- There are four ways in which neurons may react to insult:
- Acute Necrosis
- Chromatolysis
- Wallerian Degeneration
- Vacuolation
Acute Necrosis
- Acute necrosis is the most common neuronal response to injury.
- Causes of actue necrosis include:
- Ischaemia
- Diminution of the blood supply causes a lack of nutrients and oxygen, inhibiting energy production. A decrease in the levels of ATP leads to:
- Failure of the Na+/K+pumps, causing cell swelling and an increase in extracellular potassium.
- Failure to generate NAD required for DNA repair.
- Diminution of the blood supply causes a lack of nutrients and oxygen, inhibiting energy production. A decrease in the levels of ATP leads to:
- Hypoxia
- Hypoglycaemia
- Toxins, such as lead and mercury
- Ischaemia
Laminar Cortical Necrosis
- Laminar cortical necrosis refers to the selective destruction of neurons in the deeper layers of the cerebral cortex.
- These neurons are the most sensitive to hypoxia.
- The laminar cortical pattern of acute necrosis occurs in several instances:
- Ischaemia
- For example, seizure-related ischaemia in dogs.
- Polioencephalomalacia in ruminants
- Also called cerebrocortical necrosis or CCN.
- Salt poisoning in swine
- Lead poisoning in cattle
- Ischaemia
- It is most likely that gross changes will not be seen. When they are visible, changes may be apparent as:
- Oedema
- Causes brain swelling, flattened gyri and herniation
- A thin, white, glistening line along the middle of the cortex.
- In ruminants, this fluoresces with UV-light.
- Oedema
- Ultimately the cortex becomes necrotic and collapses.
View images courtesy of Cornell Veterinary Medicine
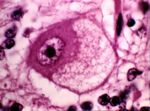
Chromatolysis
- Chromatolysis is the cell body’s reaction to axonal insult.
- The cell body swells and the Nissl substance disperses.
- Dispersal of the Nissl substance allows the cell body to produce proteins for rebuilding the axon.
- IT IS NOT A FORM OF NECROSIS.
- It is an adaptive response to deal with the injury.
- It can, however lead to necrosis.
- Seen, for example, in grass sickness in horses (equine dysautonomia).
View images courtesy of Cornell Veterinary Medicine
Wallerian Degeneration
- Wallerian degeneration is the axon’s reaction to insult.
- The axon and its myelin sheath degenerates distal to the point of injury.
- There are several causes of wallerian degeneration:
- Axonal transection
- This is the "classic" cause
- Vascular causes
- Inflamatory reactions
- Toxic insult
- As a sequel to neuronal cell death.
- Axonal transection
View images courtesy of Cornell Veterinary Medicine
The Process of Wallerian Degeneration
- Axonal Degeneration
- Axonal injuries initially lead to acute axonal degeneration.
- The proximal and distal ends separate within 30 minutes of injury.
- Degeneration and swelling of the axolemma eventually leads to formation of bead-like particles.
- After the membrane is degraded, the organelles and cytoskeleton disintegrate.
- Larger axons require longer time for cytoskeleton degradation and thus take a longer time to degenerate.
- Axonal injuries initially lead to acute axonal degeneration.
- Myelin Clearance
- Following axonal degeneration, myelin debris is cleared by phagocytosis.
- Myelin clearance in the PNS is much faster and efficient that in the CNS. This is due to:
- The actions of schwann cells in the PNS.
- Differences in changes in the blood-brain barrier in each system.
- In the PNS, the permeability increases throughout the distal stump.
- Barrier disruption in CNS is limited to the site of injury.
- Regeneration
- Regeneration is rapid in the PNS.
- Schwann cells release growth factors to support regeneration.
- CNS regeneration is much slower, and is almost absent in most species.
- This is due to:
- Slow or absent phagocytosis
- Little or no axonal regeneration, because:
- Oligodendrocytes have little capacity for remyelination compared to Schwann cells.
- There is no basal lamina scaffold to support a new axonal sprout.
- The debris from central myelin inhibits axonal sprouting.
- This is due to:
- Regeneration is rapid in the PNS.
Vacuolation
- Vacuolation is the hallmark of transmissible spongiform encephalopathies.
- For example, BSE and Scrapie.
- Vacuolation can also occur under other circumstances:
- Artefact of fixation
- Toxicoses
- It may sometimes be a normal feature.
Glial Cell Response to Injury
- The order of susceptibility of CNS cells to injury runs, from most to least susceptible:
- Neurons
- Oligodendroglia
- Astrocytes
- Microglia
- Endothelial cells
Astrocytes
- The response of astrocytes to insult include:
- Necrosis
- Astrocytosis
- An increase in the number of astrocytes (i.e. astrocyte hyperplasia).
- Astrogliosis
- An increase in the size of astrocytes (i.e. astrocyte hypertrophy).
- Gliosis
- Formation of glial fibres.
- This is a form of scarring in the CNS.
Oligodendrocytes
- Oligodendrocytes are prone to hypoxia and degeneration
- Oligodendrocytes proliferate around damaged neurons.
- This is known as satellitosis.
- Death of oligodendrocytes causes demyelination.
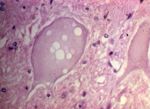
Microglial Cells
- Microglial cells can respond in two ways to CNS injury.
- They may phagocytose cell debris to transform to gitter cells.
- Gitter cells are large macrophages with foamy cytoplasm. View images courtesy of Cornell Veterinary Medicine
- They may form glial nodules.
- These are small nodules that occur notably in viral diseases.
- They may phagocytose cell debris to transform to gitter cells.
General Responses to Injury
Ischaemic Damage
- The CNS is particularly sensitive to ischaemia, because it has few energy reserves.
- The CNS is protected by its bony covering.
- Despite offering protection, the covering also makes the CNS vulnerable to certain types of damage, for example:
- Damage due to fractures and dislocation.
- Damage due to raised intracranial pressure.
- Raised intracranial stimulates a compensatory increase in blood flow, further raising intracranial pressure. This stimulates a further increase in blood flow, and the cycle continues until intracranial pressure is so high that blood flow is impeded.
- The result of this is ischaemia.
- Raised intracranial stimulates a compensatory increase in blood flow, further raising intracranial pressure. This stimulates a further increase in blood flow, and the cycle continues until intracranial pressure is so high that blood flow is impeded.
- Despite offering protection, the covering also makes the CNS vulnerable to certain types of damage, for example:
- Survival of any cell is dependent on having sufficient energy.
- Ischaemia causes cell death by impeding energy supply to cells.
- Cells directly affected by ischamia die rapidly.
- For example, those suffering a failure of pefusion due to an infarct.
- Neurons surrounding this area of complete and rapid cell death exist under sub-optimal conditions and die over a more prolonged period.
- This area of gradual death is known as the lesion penumbra.
- There are several mechanisms implicated in cell death in the penumbra:
- Increase in intracellular calcium
- Failure to control free radicals
- Generation of nitrogen species (e.g NO and ONOO) are the main damaging events.
- Cells directly affected by ischamia die rapidly.
- Ischaemia causes cell death by impeding energy supply to cells.
Oedema
- There are three types of cerebral oedema:
- Vasogenic oedema
- Vasogenic oedema follows vascular injury.
- Oedema fluid gathers outside of the cell.
- This is the most common variation of cerebral oedema.
- Cytotoxic oedema
- Cytotoxic oedema is due to an energy deficit.
- The neuron can’t pump out sodium and water leading to swelling within the cell.
- Cytotoxic oedema is due to an energy deficit.
- Interstitial oedema
- Associated with hydrocephalus.
- This type of cerebral oedema is of lesser importance.
- Vasogenic oedema
- One serious consequence of oedema is that the increase in size leads to the brain trying to escape the skull.
- This causes herniation of the brain tissue.
- The most common site of herniation is at the foramen magnum.
- The medulla is compressed at the site of the respiratory centres, leading to death.
Demyelination
- Demyelination is the loss of initially normal myelin from the axon.
- Demyelination may be primary or secondary.
Primary Demyelination
- Normally formed myelin is selectively destroyed; however, the axon remains intact.
- Causes of primary demyelination:
- Toxins, such as hexachlorophene or triethyl tin.
- Oedema
- Immune-mediated demyelination
- Infectious diseases, for example canine distemper or caprine arthritis/encephalitis.
Secondary Demyelination
- Myelin is lost following damage to the axon.
- I.e. in wallerian degeneration
Vascular Diseases
- Vascular diseases can lead to complete or partial blockage of blood flow which leads to ischaemia.
- Consequences of ischaemia depend on:
- Duration and degree of ischaemia
- Size and type of vessel involved
- Susceptibility of the tissue to hypoxia
- Consequences of ischaemia depend on:
- Potential outcomes of vascular blockage include:
- Infarct, and
- Necrosis of tissue following obstruction of its blood supply.
- Causes include:
- Thrombosis
- Uncommon in animals but may be seen with DIC or sepsis.
- Embolism. e.g.
- Bone marrow emboli following trauma or fractures in dogs
- Fibrocartilaginous embolic myelopathy
- Vasculitis, e.g.
- Hog cholera (pestivirus)
- Malignant catarrhal fever (herpesvirus)
- Oedema disease (angiopathy caused by E.coli toxin)
- Thrombosis
Malacia
- Malacia may be used:
- As a gross term, meaning "softening"
- As a microscopic term, meaning "necrosis"
- Malacia occurs in:
- Infarcted tissue
- Vascular injury, for example vasculitis.
- Reduced blood flow or hypoxia, e.g.
- Carbon monoxide poisoning, which alters hemoglobin function
- Cyanide poisoning, which inhibits tissue respiration
Excitotoxicity
- The term "excitotoxicity" is used to describe the process by which neurons are damaged by glutamate and other similar substances.
- Excitotoxicity results from the overactivation of excitatory receptor activation.
The Mechanism of Excitotoxicity
- Glutamate is the major excitatory transmitter in the brain and spinal cord.
- There are four classes of postsynaptic glutamate receptors for glutamate.
- The receptors are either:
- Directly or indirectly associated with gated ion channels, OR
- Activators of second messenger systems that result in release of calcium from intracellular stores.
- The receptors are named according to their phamacological agonists:
- NMDA receptor
- The NMDA receptor is directly linked to a gated ion channel.
- The ion channel is permeable to Ca++, as well as Na+ and K+.
- The channel is also voltage dependent.
- It is blocked in the resting state by extracellular Mg++, which is removed when membrane is depolarised.
- I.e. both glutamate and depolarisation are needed to open the channel.
- AMPA receptor
- The AMPA receptor is directly linked to a gated ion channel.
- The channel is permeable to Na+ and K+ but NOT to divalent cations.
- The receptor binds the glutamate agonist, AMPA, but is not affected by NMDA.
- The receptor probably underlies fast excitatory transmission at glutamatergic synapses.
- Kainate receptor
- Kainate receptors work in the same way as AMPA receptors, and also contribute to fast excitatory transmission.
- mGluR, the metabotropic receptor
- Metabotropic receptors are indirectly linked to a channel permeable to Na+ and K+.
- They also activate a phoshoinositide-linked second messenger system, leading to mobilisation of intra-cellular Ca++ stores.
- The physiological role ot mGluR is not understood.
- NMDA receptor
- The receptors are either:
- There are four classes of postsynaptic glutamate receptors for glutamate.
- Under normal circumstances, a series of glutamate transporters rapidly clear glutamate from the extracellular space.
- Some of these transporters are neuronal; others are found on astrocytes.
- This normal homeostatic mechanism fails under a variety of conditions, such as ischaemia and glucose deprivation.
- This results in a rise in extracellular glutamate, causing activation of the neuronal glutamate receptors.
- Two distinct events of excitiotoxicity arise from glutamate receptor activation:
- The depolarisation caused mediates an influx of Na+, Cl- and water. This give acute neuronal swelling, which is reversible.
- There is a rise in intracellular Ca++.
- This is due to:
- Excessive direct Ca++ influx via the NMDA receptor-linked channels
- Ca++ influx through voltage gated calcium channels following depolarisation of the neuron via non-NDMA receptors
- Release of Ca++ from intracellular stores.
- The rise in neuronal intracellular Ca2+ serves to:
- Uncouple mitochondrial electron transport and activate nitric oxide synthase and phospholipase A, leading to generation of reactive oxygen and nitrogen species which damage the neurone.
- Activats a number of enzymes, including phospholipases, endonucleases, and proteases.
- These enzymes go on to damage cell structures such as components of the cytoskeleton, membrane, and DNA.
- This is due to:
- Excitotoxicity is, therefore, a cause of acute neuron death.