Also Known As: ECG
Introduction
Electrocardiography is one of the most commonly used pieces of monitoring equipment in veterinary practices.
The ECG can determine the rate, rhythm and nature of cardiac depolarisation and repolarisation. It can also indicate changes in myocardial mass, conductivity between the heart and skin and the presence of metabolic abnormalities that affect the heart.
Recording an ECG
The ECG detects the electrical activity of the heart through 3 electrodes. In small animals these electrodes are most commonly placed on the 2 forelimbs and the left hindlimb. An additional electrode may be placed on the right hindlimb. Resting ECGs in the horse are recorded using a base-apex lead - with negative (red) electrode placed over the jugular groove, the positive electrode (yellow) placed just behind the left elbow over the apex beat of the heart (B) and the earth electrode attached at a site remote to the heart. This produces an ECG with large complexes that are not significantly affected by movement artifact.
The electrodes are attached to the patient via ECG pads, crocodile clips (more common in horses) and transcutaneous needles (rare). Hair should be clipped to improve contact between the ECG pad and the skin. Pads should be secured with tape and additional electrode gel or alcohol should be used to improve contact between the patient and electrodes. The electrodes are connected to the ECG machine by colour-coded cables.
The colour coding system:
- Yellow (positive): Left forelimb
- Red (negative): Right forelimb
- Green: Left hindlimb
- Black: Right hindlimb
The ECG should be recorded in a calm and quiet environment. The patient should be kept as still and relaxed as possible as muscle tremors and movement can cause artifacts on the trace. In horses the ECG is recorded standing. Dogs should be placed in right lateral recumbency but positioning in a cat is less important. The cables should be positioned so that they do not drape over the animals chest as they can cause respiratory movement artifact. Electrical activity can cause interference on the ECG trace (known as 50Hz interference). To prevent this it should be taken on an insulated surface away from fluorescent lighting, computers and other electrical equipment. A Holter ECG can be used in both small and large animal to record the electrical activity of the heart over 24 hours or during exercise.
Chemical restraint should be avoided if at all possible as this changes the ECG.
Reading an ECG Trace
An ECG only supplies information about the electrical activity of the heart. It indicates the heart rate and rhythm and can be used to detect any arrhythmias but does not supply information about cardiac function. It is important to remember to treat the patient not the ECG!
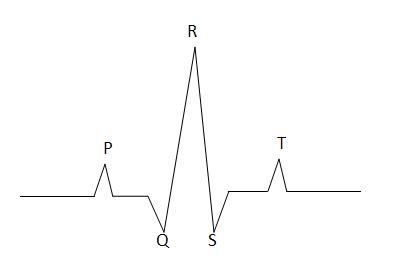
To interpret an ECG it is necessary to understand the path of depolarisation through the heart: The sino-atrial node depolarises spontaneously and this wave of depolarisation spreads through the atria. The impulse is then conducted through AV node slowly. Then it rapidly passes through the bundle of His and bundle branches, spreading through the ventricular myocardium. The myocardium then remains depolarised for a period before repolarising. This depolarisation and repolarisation are detected as potential differences on the skin surface - producing the classic shape of the ECG trace.
| Stage | Represents |
|---|---|
| P | Atrial Depolarisation |
| QRS | Ventricular Depolarisation |
| T | Ventricular Repolarisation |
In small animals six limb leads (ECG traces derived from a pair of electrodes) are produced from the reading of the four electrodes. These are named leads I, II, III, aVR, aLV, aVF.
The trace should always be read from left to right. Ideally it should be read from the start but if this is too difficult then the most recognisable portion should be identified and the trace read from this point.
A Normal ECG Trace
Before you interpret abnormal ECG's you must know what a normal ECG looks like:
- A P-wave precedes every QRS complex
- A QRS complex follows every P-wave
- P and QRS are consistently and reasonably related
- P and QRS will all appear the same
- They will occur at a normal rate
An Abnormal ECG Trace
The following questions should be asked when interpreting every ECG:
Is E.C.G. of diagnostic quality?
It is important that no artefact is present on the trace. Interference from electrical equipment and fluorescent lighting (50Hz interference) and movement should be prevented. The ECG should be calibrated for both paper speed and vertical sensitivity, and the trace should remain within the paper edges. All leads should be demonstrated.
What is the heart rate?
Heart rate can be calculated using one of the following two methods:
(1) Instantaneous heart rate
- 1500/R-R interval (25 mm/sec)
- 3000/R-R interval (50 mm/sec)
(2) Number of R-R intervals in 6 seconds x 10
Where multiple rhythms exist, the rate of all rhythms present should be calculated.
A high heart rate may be due to sinus tachycardia, supraventricular tachycardia, ventricular tachycardia and atrial fibrillation. A slow heart rate may be caused by sinus bradycardia, sinus arrhythmia, second or third degree AV block, atrial standstill and sinus arrest.
What is the heart rhythm?
It is important to interpret whether the heart rate is regular or irregular. If it is irregular you should record whether it is regularly irregular or irregularly irregular.
What is the mean electrical axis?
This figure is of limited value in small animals. It can give some indication of ventricular enlargement and the presence of intraventricular conduction defects.
What are the individual complex measurements?
Changes in the morphology of the complexes are classed as a change in the shape, size or duration of the P wave, QRS complex or T wave. The magnitude and duration of deflections can be caused by hypertrophy of the myocardium, electrolyte abnormalities or an alterations in autonomic tone within the heart.
Variation in P-wave can be caused by a wandering pacemaker or atrial ectopy. Variation in QRS complexes may be caused by either variable conduction or electrical alternans.
No P to every QRS - this occurs when ventricular depolarisation follows an abnormal atrial depolarisation. This can be caused by premature ventricular or junctional complexes, sinus arrest with ventricular or junctional escape complexes, atrial standstill and atrial fibrillation.
No QRS to every P - this occurs the AV node fails to conduct impulses normally. This can be caused by second and third degree AV block. Second degree AV block is normal in the resting horse - however it should disappear following exercise.
The shape of a complex can be used to identify the location of the origin of a rhythm disturbance. Complexes that originate in the ventricles are produce wide and bizarre QRS complexes, whereas complexes of atrioventricular origin are narrow and upright.
An abnormal P-R interval is caused by atrioventricular dissociation. This occurs secondary to junctional and ventricular rhythm disturbances and third degree AV block.
To calculate this the P-P, R-R and P-R intervals should all be measured. If the P-R interval is the only variable factor this is very suggestive of AV dissociation.
Summary
ECG is the most useful tool for assessment of cardiac rhythm. Everything possible should be done to minimise artifact and produce an ECG of good diagnostic quality. Every trace should be analysed consistently and methodically so that the results may be reliably combined with entire clinical picture.
| Electrocardiography Learning Resources | |
|---|---|
 Test your knowledge using flashcard type questions |
Feline Medicine Q&A 01 |
References
Dennis, S (2011) How to record and interpret ECGs & What is an ECG? RVC Cardiology Elective Course, Royal Veterinary College
Martin, M (2002) ECG interpretation in small animals : 1. Understanding the electricity of the heart In Practice 2002 24: 114-12
Martin, M (2002) ECG interpretation in small animals : 3. Practical guidelines In Practice 2002 24: 250-26
Menzies-Gow, N (2001) ECG interpretation in the horse In Practice 2001 23: 454-45
Robinson, SA (1990) Practical use of ECG in the horse In Practice 1990 12: 59-6
RVC staff (2009) Cardiovascular System RVC Intergrated BVetMed Course, Royal Veterinary College
Sparks, AH & Caney, SMA (2005) Self-Assessment Colour Review Feline Medicine Manson
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |