Difference between revisions of "Toxoplasmosis - Sheep"
| Line 18: | Line 18: | ||
Sheep are often kept in an environment that is significantly contaminated with oocysts, and infection follows ingestion of infected food, primarily contaminated pasture. Fields treated with manure or bedding from buildings to which cats have access result in high levels of ovine toxoplasmosis, and insecure storage of supplementary feeds also poses a risk. | Sheep are often kept in an environment that is significantly contaminated with oocysts, and infection follows ingestion of infected food, primarily contaminated pasture. Fields treated with manure or bedding from buildings to which cats have access result in high levels of ovine toxoplasmosis, and insecure storage of supplementary feeds also poses a risk. | ||
| + | |||
| + | Oocysts in the environment | ||
| + | Members of the cat family are the definitive hosts of | ||
| + | the parasite and tend to become infected for the first | ||
| + | time when they start hunting and eating wild rodents | ||
| + | and birds already infected with T. gondii. Following | ||
| + | consumption of T. gondii cysts, the parasites excyst | ||
| + | in the gut of the cat and invade and infect host cells. | ||
| + | Sexual development of the parasite takes place in the | ||
| + | gut of the cat resulting in the production of oocysts | ||
| + | which are shed in the faeces. Shedding usually occurs | ||
| + | around 3–10 days after initial infection and may | ||
| + | continue for 2–3 weeks (Dubey and Beattie, 1988). | ||
| + | During this period a cat may shed over 100 million | ||
| + | oocysts and experimental studies in sheep have shown | ||
| + | that a dose of only 200 oocysts may cause abortion in | ||
| + | previously naı¨ve pregnant sheep (McColgan, Buxton | ||
| + | and Blewett, 1988). The importance of oocysts as a | ||
| + | source of infection for sheep, has been supported by | ||
| + | studies showing an association with infection and | ||
| + | contamination of feed or grazing land with sporulated | ||
| + | oocysts (Plant, Richardson and Moyle, 1974; | ||
| + | Faull, Clarkson and Winter, 1986) and also work | ||
| + | showing an association with cats on farms and | ||
| + | prevalence of T. gondii in sheep (Skjerve et al. 1998). | ||
| + | Further studies looking at development of specific | ||
| + | antibodies in sheep, as an indicator of exposure to | ||
| + | T. gondii, have shown that there is an increase in seroprevalence | ||
| + | associated with age. This indicates that | ||
| + | there is extensive environmental contamination with | ||
| + | T. gondii oocysts and that most infections in sheep | ||
| + | occur following exposure to the parasite after birth | ||
| + | (Waldeland, 1977; Blewett, 1983; Lunden,Nasholm | ||
| + | and Uggla, 1994). Recent studies have indicated that | ||
| + | there is widespread environmental contamination | ||
| + | with T. gondii oocysts (Dabritz et al. 2007). | ||
| + | Congenital transmission | ||
| + | Primary infection during pregnancy. As sheep are | ||
| + | not carnivores, consumption of tissues infected with | ||
| + | T. gondii bradyzoites contained within tissue cysts | ||
| + | is not considered to be a route of transmission in | ||
| + | these animals. The only other route of transmission | ||
| + | is vertical from mother to foetus during pregnancy | ||
| + | (Buxton and Rodger, 2008). The stage of pregnancy | ||
| + | when transplacental transmission of T. gondii takes | ||
| + | place is important in determining the clinical outcome. | ||
| + | If infection occurs early in gestation, when the | ||
| + | foetal immune system is relatively immature, foetal | ||
| + | death is likely to occur. Infection at mid-gestation | ||
| + | can result in birth of a stillborn or weak lamb which | ||
| + | may have an accompanying small mummified foetus, | ||
| + | whereas infection in later gestation may result in | ||
| + | birth of a live, clinically normal, but infected lamb | ||
| + | (Buxton, 1990). The birth of clinically normal but | ||
| + | infected lambs usually occurs as a result of a primary | ||
| + | infection in the third trimester of pregnancy, | ||
| + | although it is also possible that transplacental transmission | ||
| + | may occur as a result of recrudescence of an | ||
| + | endogenous infection (Trees and Williams, 2005). | ||
| + | Recrudescence of an endogenous infection. While recrudescence | ||
| + | of a persistent endogenous infection is | ||
| + | a very common route of congenital infection with | ||
| + | the closely related parasiteNeospora caninum in cattle | ||
| + | (Innes et al. 2005; Williams et al. 2009 – this special | ||
| + | issue), it is not thought to be a significant route of | ||
| + | transmission for T. gondii infection in sheep (Dubey | ||
| + | and Beattie, 1988; Buxton and Rodger, 2008). | ||
| + | However, recent studies, (Duncanson et al. 2001; | ||
| + | Williams et al. 2005, Morley et al. 2005, 2008), have | ||
| + | suggested that endogenous transplacental transmission | ||
| + | of T. gondii may be more important than | ||
| + | was previously thought and that this route of transmission | ||
| + | may be an important cause of lamb mortality. | ||
| + | Data reported by Williams et al. (2005) stated | ||
| + | that 53.7% of lambs in their test flocks had evidence | ||
| + | of congenital T. gondii infection at birth with 46% | ||
| + | of live lambs and 90% of dead lambs being positive | ||
| + | for T. gondii by PCR analysis. Further work that | ||
| + | followed ewes over successive pregnancies reported | ||
| + | a frequency of 21% for successive T. gondii positive | ||
| + | abortions, suggesting that complete protective immunity | ||
| + | has not been acquired following a previous | ||
| + | infection (Morley et al. 2008). | ||
| + | These studies are very interesting although difficult | ||
| + | to interpret with confidence as they rely heavily | ||
| + | on PCR-based techniques and the methodology is | ||
| + | not validated using supporting pathology, serological | ||
| + | evidence or isolation of live parasites to show that | ||
| + | the live lambs in the study were indeed congenitally | ||
| + | infected with T. gondii as a result of endogenous | ||
| + | transmission. In addition, the authors did not rule | ||
| + | out other causes of abortion due to different pathogens | ||
| + | on their study farm. These studies also raise | ||
| + | the importance of the language we use to describe | ||
| + | vertical transmission. To aid our understanding of | ||
| + | this area it is important to define the difference | ||
| + | between endogenous transplacental transmission and | ||
| + | exogenous transplacental transmission as described | ||
| + | by Trees and Williams (2005). | ||
| + | A recent relevant study in this area using a full | ||
| + | range of different diagnostic techniques found that, | ||
| + | in contrast to the studies described above, there was | ||
| + | no significant transmission from persistently infected | ||
| + | sheep to their offspring (Rodger et al. 2006). In this | ||
| + | study, a group of sheep previously infected with | ||
| + | Elisabeth A. Innes and others 1888 | ||
| + | T. gondii and a group of naı¨ve control sheep were | ||
| + | mated and followed through pregnancy to lambing. | ||
| + | A full post-mortem was conducted on any dead lambs | ||
| + | and placentas were examined using histopathological | ||
| + | techniques and by T. gondii-specific PCR for evidence | ||
| + | of infection. In addition, pre-colostral blood | ||
| + | samples were collected from all the lambs to look | ||
| + | for antibodies to T. gondii. The presence of T. gondii | ||
| + | antibodies in pre-colostral blood samples is a good | ||
| + | indicator that congenital transmission has occurred. | ||
| + | The results showed that the group of 31 T. gondiiinfected | ||
| + | sheep gave birth to 43 live healthy lambs | ||
| + | and 6 stillborn lambs. There was no evidence of | ||
| + | T. gondii infection in any of the tissues examined | ||
| + | using T. gondii-specific PCR and histopathological | ||
| + | techniques, in addition all the foetal fluid samples | ||
| + | from the dead lambs and the pre-colostral serum | ||
| + | samples from the live lambs were sero-negative with | ||
| + | the exception of one set of twin lambs born to one | ||
| + | of the infected ewes. All the T. gondii-negative ewes | ||
| + | produced live T. gondii-negative lambs. Therefore | ||
| + | this more complete study using a variety of scientific | ||
| + | techniques to confirm transmission and infection | ||
| + | showed that the rate of congenital transmission from | ||
| + | persistently infected ewes was very infrequent, | ||
| + | around 3.2% (Rodger et al. 2006). | ||
| + | Data from previous published papers in this area | ||
| + | also agree with the results of Rodger et al. that | ||
| + | although endogenous transplacental transmission of | ||
| + | T. gondii may occur it is very infrequent and does not | ||
| + | pose a significant clinical risk. A study by Watson | ||
| + | and Beverley in the UK showed that in a group of 26 | ||
| + | ewes that were infected in a previous pregnancy with | ||
| + | T. gondii and then retained and followed through a | ||
| + | subsequent pregnancy gave birth to 24 live uninfected | ||
| + | lambs with only one ewe aborting a pair | ||
| + | of twins (Watson and Beverley, 1971). A larger study | ||
| + | in Australia examined what proportion of lambs may | ||
| + | be infected as a result of a re-activation of a previous | ||
| + | infection and found that a group of 135 persistently | ||
| + | infected ewes produced 178 live lambs all being precolostral | ||
| + | antibody negative with evidence of only one | ||
| + | of the ewes having an infected placenta. In addition, | ||
| + | there was no evidence of T. gondii being isolated from | ||
| + | their tissues using mouse inoculation. Therefore they | ||
| + | concluded that congenital transmission of T. gondii | ||
| + | from ewes persistently infected with the parasite is | ||
| + | very infrequent (Munday, 1972).. | ||
==Signalment== | ==Signalment== | ||
Revision as of 15:13, 13 August 2010
| This article is still under construction. |
Description
Toxoplasmosis is the disease caused by Toxoplasma gondii, an intracelluler protozoan parasite. Although the definitive host is the cat, T. gondii can infect all mammals including man and is a significant cause of abortion in sheep and goats. Toxoplasmosis does not seem to cause disease in cattle.
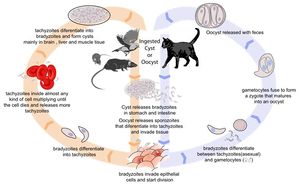
Life Cycle
There are three infectious stages of Toxoplasma gondii: 1) sporozoites; 2) actively reproducing tachyzoites; and 3) slowly multiplying bradyzoites. Tachyzoites and bradyzoites are found in tissue cysts, whereas sporozoites are containted within oocysts, which are excreted in the faeces. This means that the protozoa can be transmitted by ingestion of oocyst-contaminated food or water, or by consumption of infected tissue.
In naive cats, Toxoplasma gondii undergoes an enteroepithelial life cycle. Cats ingests intermediate hosts containing tissue cysts, which release bradyzoites in the gastrointestinal tract. The bradyzoites penetrate the small intestinal epithelium and sexual reproductio ensues, eventually resulting the production of oocysts. Oocysts are passed in the cat's faeces and sporulate to become infectious once in the environment. These can then be ingested by other mammals, including sheep.
When sheep ingest oocysts, T.gondii intiates extraintestinal replication. This process is the same for all hosts, and also occurs when carnivores ingest tissue cysts in other animals. Sporozoites (or bradyzoites, if cysts are consumed) are released in the intestine to infect the intestinal epithelium where they replicate. This produces tachyzoites, which reproduce asexually within the infected cell. When the infected cell ruptures, tachyzoites are released and disseminate via blood and lymph to infect other tissues. Tachyzoites then replicate intracellularly again and the process continues until the host becomes immune or dies. If the infected cell does not burst, tachyzoites eventually encyst as bradyzoites and persist for the life of the host. Cyst are most commonly found in the brain or skeletal muscle, and are a source of infection for carnivorous hosts.
Transmission to Sheep
Infected cats shed oocysts continuously between days 3 and 14 post-infection. During this time, hundreds of millions of oocysts may be shed. The main sources of feline toxoplasma infection are chronically infected birds and rodents. Rodents are particularly important since they can pass T. gondii infection to their offspring without causing clinical disease. This means that a farm may develop a reservoir of T. gondii tissue cysts with the potential to cause feline infection and massive oocyst excretion. In turn, environments may easily become contaminated with a high oocyst burden when a cat is introduced.
Sheep are often kept in an environment that is significantly contaminated with oocysts, and infection follows ingestion of infected food, primarily contaminated pasture. Fields treated with manure or bedding from buildings to which cats have access result in high levels of ovine toxoplasmosis, and insecure storage of supplementary feeds also poses a risk.
Oocysts in the environment Members of the cat family are the definitive hosts of the parasite and tend to become infected for the first time when they start hunting and eating wild rodents and birds already infected with T. gondii. Following consumption of T. gondii cysts, the parasites excyst in the gut of the cat and invade and infect host cells. Sexual development of the parasite takes place in the gut of the cat resulting in the production of oocysts which are shed in the faeces. Shedding usually occurs around 3–10 days after initial infection and may continue for 2–3 weeks (Dubey and Beattie, 1988). During this period a cat may shed over 100 million oocysts and experimental studies in sheep have shown that a dose of only 200 oocysts may cause abortion in previously naı¨ve pregnant sheep (McColgan, Buxton and Blewett, 1988). The importance of oocysts as a source of infection for sheep, has been supported by studies showing an association with infection and contamination of feed or grazing land with sporulated oocysts (Plant, Richardson and Moyle, 1974; Faull, Clarkson and Winter, 1986) and also work showing an association with cats on farms and prevalence of T. gondii in sheep (Skjerve et al. 1998). Further studies looking at development of specific antibodies in sheep, as an indicator of exposure to T. gondii, have shown that there is an increase in seroprevalence associated with age. This indicates that there is extensive environmental contamination with T. gondii oocysts and that most infections in sheep occur following exposure to the parasite after birth (Waldeland, 1977; Blewett, 1983; Lunden,Nasholm and Uggla, 1994). Recent studies have indicated that there is widespread environmental contamination with T. gondii oocysts (Dabritz et al. 2007). Congenital transmission Primary infection during pregnancy. As sheep are not carnivores, consumption of tissues infected with T. gondii bradyzoites contained within tissue cysts is not considered to be a route of transmission in these animals. The only other route of transmission is vertical from mother to foetus during pregnancy (Buxton and Rodger, 2008). The stage of pregnancy when transplacental transmission of T. gondii takes place is important in determining the clinical outcome. If infection occurs early in gestation, when the foetal immune system is relatively immature, foetal death is likely to occur. Infection at mid-gestation can result in birth of a stillborn or weak lamb which may have an accompanying small mummified foetus, whereas infection in later gestation may result in birth of a live, clinically normal, but infected lamb (Buxton, 1990). The birth of clinically normal but infected lambs usually occurs as a result of a primary infection in the third trimester of pregnancy, although it is also possible that transplacental transmission may occur as a result of recrudescence of an endogenous infection (Trees and Williams, 2005). Recrudescence of an endogenous infection. While recrudescence of a persistent endogenous infection is a very common route of congenital infection with the closely related parasiteNeospora caninum in cattle (Innes et al. 2005; Williams et al. 2009 – this special issue), it is not thought to be a significant route of transmission for T. gondii infection in sheep (Dubey and Beattie, 1988; Buxton and Rodger, 2008). However, recent studies, (Duncanson et al. 2001; Williams et al. 2005, Morley et al. 2005, 2008), have suggested that endogenous transplacental transmission of T. gondii may be more important than was previously thought and that this route of transmission may be an important cause of lamb mortality. Data reported by Williams et al. (2005) stated that 53.7% of lambs in their test flocks had evidence of congenital T. gondii infection at birth with 46% of live lambs and 90% of dead lambs being positive for T. gondii by PCR analysis. Further work that followed ewes over successive pregnancies reported a frequency of 21% for successive T. gondii positive abortions, suggesting that complete protective immunity has not been acquired following a previous infection (Morley et al. 2008). These studies are very interesting although difficult to interpret with confidence as they rely heavily on PCR-based techniques and the methodology is not validated using supporting pathology, serological evidence or isolation of live parasites to show that the live lambs in the study were indeed congenitally infected with T. gondii as a result of endogenous transmission. In addition, the authors did not rule out other causes of abortion due to different pathogens on their study farm. These studies also raise the importance of the language we use to describe vertical transmission. To aid our understanding of this area it is important to define the difference between endogenous transplacental transmission and exogenous transplacental transmission as described by Trees and Williams (2005). A recent relevant study in this area using a full range of different diagnostic techniques found that, in contrast to the studies described above, there was no significant transmission from persistently infected sheep to their offspring (Rodger et al. 2006). In this study, a group of sheep previously infected with Elisabeth A. Innes and others 1888 T. gondii and a group of naı¨ve control sheep were mated and followed through pregnancy to lambing. A full post-mortem was conducted on any dead lambs and placentas were examined using histopathological techniques and by T. gondii-specific PCR for evidence of infection. In addition, pre-colostral blood samples were collected from all the lambs to look for antibodies to T. gondii. The presence of T. gondii antibodies in pre-colostral blood samples is a good indicator that congenital transmission has occurred. The results showed that the group of 31 T. gondiiinfected sheep gave birth to 43 live healthy lambs and 6 stillborn lambs. There was no evidence of T. gondii infection in any of the tissues examined using T. gondii-specific PCR and histopathological techniques, in addition all the foetal fluid samples from the dead lambs and the pre-colostral serum samples from the live lambs were sero-negative with the exception of one set of twin lambs born to one of the infected ewes. All the T. gondii-negative ewes produced live T. gondii-negative lambs. Therefore this more complete study using a variety of scientific techniques to confirm transmission and infection showed that the rate of congenital transmission from persistently infected ewes was very infrequent, around 3.2% (Rodger et al. 2006). Data from previous published papers in this area also agree with the results of Rodger et al. that although endogenous transplacental transmission of T. gondii may occur it is very infrequent and does not pose a significant clinical risk. A study by Watson and Beverley in the UK showed that in a group of 26 ewes that were infected in a previous pregnancy with T. gondii and then retained and followed through a subsequent pregnancy gave birth to 24 live uninfected lambs with only one ewe aborting a pair of twins (Watson and Beverley, 1971). A larger study in Australia examined what proportion of lambs may be infected as a result of a re-activation of a previous infection and found that a group of 135 persistently infected ewes produced 178 live lambs all being precolostral antibody negative with evidence of only one of the ewes having an infected placenta. In addition, there was no evidence of T. gondii being isolated from their tissues using mouse inoculation. Therefore they concluded that congenital transmission of T. gondii from ewes persistently infected with the parasite is very infrequent (Munday, 1972)..
Signalment
Diagnosis
Clinical Signs
- Clinical outbreaks of toxoplasmosis are sporadic
- Immunity is acquired before tupping
- Significant ill-effects are unlikely if immune ewes are infected during pregnancy
- Not shed from sheep to sheep so predicting outbreaks is difficult
Laboratory Tests
Pathology
Aborted ewes show focal necrotic placentitis with white lesions in the cotyledons and foetal tissue
Treatment
- Toxovax vaccine
- Live, avirulent strain of Toxoplasma
- Does not form bradyzoites or tissue cysts
- Killed by host immune system
- Single dose given 6 weeks before tupping
- Protects for 2 years
- Immunity boosted by natural challenge
- Medicated feed can be given daily during the main risk period
- 14 weeks before lambing
- The best method of protection is to prevent cats from contaminating the pasture, lambing sheds and feed stores
The extent of environmental contamination with T. gondii oocysts is thus related to the distribution and behaviour of cats. Measures to reduce environmental contamination by oocysts should be aimed at reducing the number of cats capable of shedding oocysts. This would include attempts to limit their breeding. If male cats are caught, neutered and returned to their colonies the stability ofthe colony is maintained; fertile male cats do not challenge the neutered males12 and breeding is controlled. Thus the maintenance ofa small healthy population of mature cats will reduce oocyst excretion as well as help to control rodents. Sheep feed should be kept covered at all times to prevent its contamination by cat faeces.
Prognosis
Links
References
- Buxton, D (1990) Ovine toxoplasmosis: a review. Journal of the Royal Society of Medicine, 83, 509-511.
- Innes, E A et al (2009) Ovine toxoplasmosis. Parastiology, 136, 1887–1894.
- Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. Veterinary Parasitology, 147, 25-28.
- Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. Veterinary Parasitology, 163, 1-14.