Eye Examination - Donkey
The equipment, assessment and use of diagnostic techniques for examination of the donkey eye are the same as for a horse.
Essential equipment
- Storage box. A fishing tackle box is ideal for keeping kit dry and clean.
- Focal light. Ideally a Finoff transilluminator, direct ophthalmoscope head and rechargeable hand piece.
- Fluorescein paper or drops. Apply to any eye that appears inflamed, cloudy or painful. Wipe away any excess. Apply before any local anaesthetic.
- Schirmer tear test strips.
- Mydriatic, 1% Tropicamide.
- Topical anaesthetic, 0.5% proxymetacaine hydrochloride.
- Artificial tears. To clean debris from the eye and provide a vehicle for EDTA.
- Irrigating cannula for nasolacrimal cannulation, 0.76 mm or 0.91 mm
- Sterile transport swabs for corneal ulcers and refractive conjunctivitis.
- Local anaesthetic for nerve blocks, 2% lignocaine.
- Sedatives, alpha2-agonists +/- butorphanol.
- NSAIDs, flunixin meglumine, phenylbutazone.
Differential Diagnoses
History-taking is an important part of making a list of differential diagnoses. Completing an ophthalmic examination sheet helps form a complete picture. Recording the ocular disorder using drawings allows accurate comparisons to be made at follow-up examinations.
Chemical restraint for examination
Although the donkey is more stoical than the horse, the ocular muscles are still too strong to allow easy, thorough examination of the eye. In the case of blepharospasm, regional anaesthesia is an essential tool. Sedation is helpful in the case of a fractious animal or to facilitate treatment, e.g. subconjunctival injections, contact lens placement.
Sedation
Romifidine in combination with butorphanol is most frequently used with reliable results. Detomidine is used less, due to the tiny volumes being handled and the increased ataxia.
Frontal/supraorbital nerve block
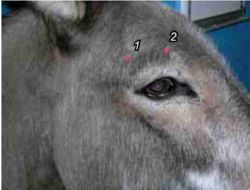
This block is used most commonly to allow a thorough ophthalmic examination. It blocks sensation to the central two-thirds of the upper eyelid and is useful alone. It is essential prior to installation of a subpalpebral lavage system.
Method: Clip and aseptically prepare the area above the eye. Inject 3-4 ml of 2% lignocaine into the supraorbital foramen of the frontal bone, using a 25G 16 mm needle. Inject 1-2 ml, with the needle inserted to the hub, then 1-2 ml subcutaneously as the needle is withdrawn. The foramen is easy to feel with the fingernail but can be hard to find when attempting to fully insert the needle. It may involve redirecting the tip of the needle until it passes through the foramen. Always withdraw to check for blood.
Auriculopalpebral nerve block
This block provides loss of motor function of the upper eyelid five to ten minutes after anaesthesia of the dorsal and ventral branches of the palpebral nerve as it passes between the vertical ramus of the mandible and the zygomatic process of the temporal bone. The nerve cannot be palpated but a natural depression marks the site at the intersection of two lines, one drawn parallel to the caudal aspect of the ramus and the other parallel to the dorsal aspect of the zygomatic process.
Method: Clip and aseptically prepare the area before inserting a 25G 16 mm needle. Inject 4 ml of 2% lignocaine, redirecting the needle to maximise infiltration
Other nerve blocks
Less commonly used are three other nerve blocks:
- for the zygomatic nerve, which provides sensation to the central two-thirds of the lower eyelid;
- for the infratrochlear nerve, which supplies the medial canthus;
- for the lacrimal nerve, which supplies the lateral canthus and lateral aspect of the upper lid.
Topical anaesthesia
Proxymetacaine 0.5%, applied directly to the eye, desensitises the cornea and conjunctiva. It is required in many cases to relieve discomfort and allow examination, and for sample collection or treatment. It also allows examination and handling of the nictitating membrane. If topical anaesthesia is used to collect samples for cytology, it can affect the staining. Schirmer tear tests and sample collection should preferably be performed prior to applying any stains or medications to the eye.
Literature Search
Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation).
Ocular examination in donkeys or horses publications
References
- Grove, V. (2008) Conditions of the eye In Svendsen, E.D., Duncan, J. and Hadrill, D. (2008) The Professional Handbook of the Donkey, 4th edition, Whittet Books, Chapter 11
|
|
This section was sponsored and content provided by THE DONKEY SANCTUARY |
|---|
