Fine needle aspiration techniques
Fine needle aspirates (FNA) are inexpensive and can be collected quickly and easily with little or no risk to the patient. Ultrasound guided aspirates allow sampling of focal
lesions and thoracic and abdominal organs or masses may be sampled with less risk of complications than for conventional core biopsy techniques. When using ultrasound guidance it is important that ultrasound gel (which may obscure all cellular detail in an aspirate) is removed prior to needle insertion.
Possible complications include haemorrhage, peritonitis, bacteraemia, fistula formation and tumour seeding but the risk is reported to be low for abdominal aspirates and in most cases is outweighed by the diagnostic benefit. However, evaluation for a bleeding disorder is recommended, particularly if a vascular structure is to be aspirated and there are some circumstances in which FNA is contraindicated (see below).
Cytological specimens collected by FNA lack the cellular architecture necessary to characterise many lesions as completely as by full tissue biopsy. FNA frequently helps to determine the type of pathologic process (inflammatory, neoplastic etc) but biopsy may be necessary to fully characterise a neoplastic process and therefore to determine the prognosis.
Lymph nodes
Fine needle aspirates of enlarged peripheral lymph nodes can usually be obtained without anaesthesia or sedation. A 23-25g 1-1½” needle and 5ml syringe can be used, or a needle alone. The needle-only technique may reduce haemodilution of the sample and lysis of fragile cells caused by aspiration.
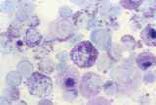
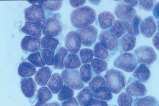
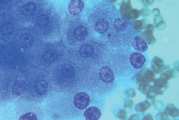
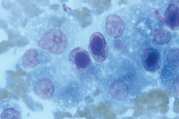
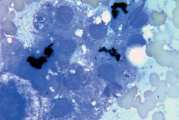
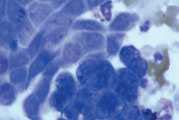
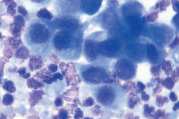
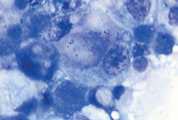
First, swab the skin over the lymph node with alcohol. The lymph node is fixed between thumb and forefinger and the needle introduced. When using a syringe, negative pressure is applied whilst moving the needle through the lymph node to obtain a representative sample. Negative pressure is released before withdrawing the needle. Disconnect the needle and syringe, draw air into the syringe, re-attach, and carefully expel the sample onto two clean glass slides. Smears are then prepared using a blood smear technique (Fig 1), or gentle squash technique (Fig 2). Lymphoma cells are fragile and the blood smear technique often results in less cellular lysis than the alternative.
For the needle-only technique, a 25g needle is inserted into the lymph node and redirected/ retracted several times. Cells are collected by capillary action. The needle is withdrawn, a syringe containing air connected and the sample gently expelled onto slides. Smears are prepared as described above.
Cytological examination is most likely to be diagnostic in cases of lymphoma or metastatic neoplasia in which there is extensive involvement of the node. The submandibular lymph node is often reactive and should be avoided if there are other nodes which might be sampled. Reactive hyperplasia, lymphadenitis and many cases of lymphoma can be diagnosed with confidence on good quality aspirates but where there is doubt as to the diagnosis, then histopathology will be recommended to establish a diagnosis.
Liver
Indications
Fine needle aspiration of the liver has limited diagnostic utility and is predominantly used in the investigation of diffuse hepatomegaly or focal lesions. It may be useful for diagnosis of lymphoma and for identification of cytoplasmic change consistent with hepatic lipidosis and vacuolar hepatopathy. However, the technique is less useful for diagnosis of inflammatory hepatopathies and differentiation of benign and malignant hepatocellular or bile duct neoplasms.
The correlation between cytologic examination of fine-needle liver aspirates and histological examination of hepatic biopsy samples is reported to be poor. The diagnostic correlation of samples was 30% in dogs and 51% in cats in one study.
For diffuse hepatomegaly, a representative sample can be obtained without visual guidance (if ultrasound is not available) but focal lesions require visualisation (ultrasonography, laparoscopy). If a laparotomy is performed then a biopsy for histology is usually more appropriate. If larger tissue biopsies are taken, then impression smears of the biopsy surface may be made prior to fixation and allow for more rapid examination.
Contraindications
FNA is contra-indicated if there is any evidence of defective haemostasis. Although FNA cytology causes minimal tissue damage, platelets are most important in occluding defects in vessels. The platelet count should be >10 per field using the x100 oil objective, assessed in the monolayer of the smear. Coagulation studies are recommended prior to FNA or other biopsy techniques although some texts state that a coagulation screen is unnecessary if there was no haemorrhage associated with venipuncture. Aspiration techniques are also contraindicated where haemangiosarcoma is suspected. FNA of such lesions is unlikely to provide a diagnosis and risks rupture of the capsule.
Equipment
- A needle with a stylet is preferable (for example a 2½” spinal needle with a stylet) as the stylet prevents obstruction of the needle by tissue from the body wall. The stylet is removed when the liver has been penetrated. A 1½” 22g hypodermic needle is adequate if a spinal needle is not available
- A 2-5ml syringe
- Clean glass slides
Blind aspiration technique
Ultrasound guided sampling is preferred but if this is not possible a blind aspiration technique may be used. Fine needle aspiration of the liver can be performed without sedation, depending on the temperament of the patient. Two alternative approaches are described:
Standing
- Restrain the patient in a standing position
- Prepare aseptically the 11th intercostal space on the RHS
- Only 1-2ml of negative pressure should be applied Dorsal recumbency
- Position the patient in dorsal recumbency with the body tilted slightly to the right and the head elevated (causes the liver to move caudally within the abdominal cavity)
- Prepare aseptically a region on the left side including the xiphoid process medially and the costal arch laterally
- Insert the needle midway between the left costal arch and the end of the xiphoid process at an angle of approximately 30-45 degrees to the skin. In cats the angle of insertion must be almost vertical to avoid penetrating the diaphragm
- Advance the needle and after the liver has been penetrated apply about 5ml negative pressure as the needle is advanced through the liver. If blood appears in the syringe release the negative pressure, if aspiration is continued blood can dilute the sample and may clot, making it difficult to prepare smears
- Gently release the suction before removing the needle from the abdominal cavity
- Prepare smears by the routine blood smear technique (Fig 1) and rapidly air dry. If possible prepare several smears. Do not fix or cover-slip
- If the sample contains too few cells, repeat
Lung
Trans-thoracic fine needle aspiration may be considered if less invasive procedures, for example trans-tracheal or bronchial wash have not, or are unlikely to provide diagnostic material. Pulmonary aspirates can be obtained quickly and the likelihood of obtaining a useful or diagnostic sample is high particularly if accurate needle placement can be determined. Diffuse lesions often yield high quality samples while in generalised lung disease, multiple attempts may be required due to the aspiration of normal cellular components or non-representative areas. A definitive diagnosis may not be achieved but characterisation of inflammation versus neoplasia is often possible.
In very sick patients, FNA of the lung may be used to avoid higher-risk procedures such as thoracotomy or biopsy.
Indications
To investigate the aetiology of a large, solitary pulmonary mass, an area of consolidation, or a diffuse disease of the parenchyma. Complications include haemorrhage and pneumothorax. Coagulation screening (prothrombin time, activated partial thromboplastin time, platelet count) prior to sampling is recommended. It is reported that the risk of complications increases proportionally with the duration of the procedure
Contraindications
- Uncooperative patient - possible laceration of the lung
- Uncontrollable coughing or dyspnoea - possible laceration of the lung
- Any bleeding disorder - may cause severe haemorrhage
- Pulmonary hypertension - may cause severe bleeding
- Pulmonary cysts or bullous emphysema - may cause severe pneumothorax
Equipment
- 23-25g, 1-2” needle, with a 2-5ml syringe attached
- Clean glass slides
Method
Ultrasound guided aspiration is preferred and allows sampling from localised lesions. If ultrasonography is not available then a blind aspiration technique may be considered for diffuse pathology. In this technique the needle is inserted on the right side of the chest between 7th and 9th ribs two thirds of the way dorsally between the costochondral junction and the vertebral bodies.
- Restrain the patient in sternal recumbency or standing. Heavy sedation may be needed for uncooperative patients, but should be avoided if possible. Sedated patients cannot clear respiratory secretions or bronchial haemorrhage which may occur during FNA sampling
- Clip the hair over the area. Scrub, drape and apply antiseptic
- Local anaesthetic may be injected into the anterior edge of the intercostal space
- Insert the needle with attached syringe aseptically through the chest wall at the cranial aspect of the rib, to avoid the intercostal vessels on the adjacent rib’s caudal border
- Temporarily hold the nose and mouth closed to stop lung movement while the needle is advanced into the lung (1-3cm) and apply negative pressure while the needle is moved backwards and forwards. Changing the angle of the needle is likely to increase the risk of complications although will also increase the likelihood of collecting a representative sample
- Release the negative pressure and withdraw the needle
- Remove the needle from the syringe and fill the syringe with air
- Reconnect the needle to the syringe and express the contents of the needle onto one or more clean glass slides. Smear the material and rapidly air dry. If possible prepare several smears. If the sample contains too few cells, repeat
- Post aspiration care. Closely monitor the patient for complications. Pneumothorax is the most common complication occurring in about 20% of cases. Most, however, are mild and asymptomatic and resolve spontaneously. Rotating the animal so that the puncture site is on the dependent side for 3 minutes decreases the risk of pneumothorax formation. The clinician should be prepared to insert a chest drain if severe pneumothorax occurs. Thoracic radiographs are recommended at 1 hour and 24 hours after aspiration
Spleen
Indications
FNA is useful for evaluation of diffuse splenomegaly, such as might be associated with hyperplasia (infection or immunologic disease), extramedullary haematopoiesis, lymphoproliferative disease and haematopoietic neoplasia. One study reports that the cytologic diagnoses in aspirates from 31 dogs and cats corresponded with histological diagnoses in 19 cases (61.3%) but that histological examination of the tissue architecture may be required to differentiate between reactive and neoplastic pathologies.
Contraindications
Aspiration is contraindicated where there is defective haemostasis and when haemangiosarcoma is suspected. Sampling should not be attempted when there is asymmetric splenomegaly or vascular pathology which may be a consequence of haematoma, splenic neoplasia (haemangioma, haemangiosarcoma, fibrosarcoma, leiomyosarcoma) and metastatic neoplasia.
Equipment
- 22-23g, 1-1½” needle, with a 5-10ml syringe attached
- Clean glass slides
Method
Ultrasound guided aspiration is preferred and allows sampling from localised lesions. If ultrasonography is not available then a blind aspiration technique may be considered for diffuse pathology. Aspiration of the spleen usually does not require general anaesthesia but the patient must be adequately restrained or sedation to minimise the risk of haemorrhage. The site of aspiration depends on the size and location of the spleen. Select the site where the spleen can be most easily apposed to the abdominal wall.
- The patient is restrained in right lateral or dorsal recumbency
- Clip and prepare the site as for any aseptic procedure. Infiltration with local anaesthetic may be helpful
- Press the spleen gently against the abdominal wall at the prepared site
- Insert the needle with syringe attached through the skin and abdominal wall into the spleen
- Apply negative pressure while moving the needle within the spleen and redirecting along several axes. It has been suggested that a non-aspirational technique reduces the risk of blood contamination and increases cellularity in canine splenic aspirates. Both techniques may be attempted
- Release negative pressure when a small amount of bloody fluid appears in the syringe. Withdraw the needle
- Gently express the aspirate onto a glass slide. Usually the aspirate has the consistency of blood and slides can be prepared in the same way as for blood smears. If tissue fragments are present prepare by the squash technique (Fig 2)
- Smear the material and rapidly air dry. If possible prepare several smears
Kidney
Cytological examination is often limited by poor cellular exfoliation and cellular disruption, however, FNA often provides a diagnosis in patients with renal lymphoma. Multiple aspirates, at least 4 or 5 have been reported to improve the chance of obtaining a diagnostic sample. Postaspiration haemorrhage is the major reported complication and a coagulation screen prior to sampling should be considered.
Indications
Renomegaly caused by diffuse enlargement, localised mass or fluid accumulation.
Contraindications
- Any bleeding disorder
- Distended loops of bowel
- Patient disposition preventing adequate restraint during sampling
Equipment
- 23-25g, 1-1½” needle, with a 5-10ml syringe attached
- Clean glass slides
Method
Ultrasound guided aspiration is preferred and allows sampling from localised lesions. If ultrasonography is not available then a blind aspiration technique may be considered for cats and sometimes dogs, when the kidney can be immobilised against the abdominal wall. If the changes are diffuse then sampling from the caudal pole of the left kidney is recommended. Sedation or anaesthesia may be required for adequate restraint, to prevent sudden movement during aspiration.
- Clip and aseptically prepare the site over which the kidney is to be positioned
- Place the patient in lateral recumbency and manually immobilise the kidney against the body wall
- Insert the needle with syringe attached through the body wall into the kidney. If the lesion is focal then direct the needle into it. If the kidney is diffusely enlarged then direct the needle into the cortex. Avoid the hilar region containing the renal artery and vein
- Apply negative pressure while the syringe is moved back and forth, about 1cm, several times. If blood is aspirated the procedure should be stopped
- Release negative pressure before the needle is withdrawn
- Detach the syringe and fill with air, reattach and express material onto a glass slide
- Smear the material and rapidly air dry. If possible prepare several smears
- If fluid is aspirated then place in an EDTA tube and make both direct smears and smears of sediment after fluid centrifugation
Prostate
FNA of the prostate gland often yields more prostatic cells than other methods of sample collection. However, material can be obtained by several techniques including:
- Digital massage while aspirating through a urinary catheter passed to the level of the prostate
- Washing the prostatic urethra by gently injecting and aspirating a small amount of saline. Cells can then be concentrated by centrifugation
- Inducing ejaculation, the first fraction of the ejaculate contains prostatic fluid
Indications
Aspiration is indicated in the investigation of prostatomegaly (symmetrical, unilateral enlargement or focal irregularities).
Contraindications
- Bleeding disorders
- Suspected prostatic abscess or acute prostatitis. In these cases there may be an increased risk of peritonitis associated with direct FNA and collection of material by prostatic massage/wash is recommended
Equipment
- 22-23g, 1-1½” needle, with a 10ml syringe attached
- Clean glass slides
Method
Trans-abdominal or trans-perineal/peri-rectal aspiration may be used. Ultrasound guided aspiration is preferred and allows sampling from localised lesions. If ultrasonography is not available then a blind aspiration technique may be considered for diffuse pathology.
- Prepare the skin at either site as for any aseptic surgical procedure. Use local anaesthesia of the skin to prevent discomfort and to make it easier to direct the needle. This is usually needed with the peri-rectal technique
- For a trans-abdominal approach, place the dog in dorsal recumbency. Elevate the prostate using rectal digital palpation and pass the needle into the visible bulge in the caudoventral abdominal wall
- For a peri-rectal approach, pass the needle through the skin in the perineal region and guide to the prostate along a finger in the rectum
- Gently aspirate the gland while moving the needle within the prostate. If purulent material is aspirated then continue aspirating until no more material is collected in an attempt to reduce the fluid pressure and the risk of leakage of exudate into the peritoneal cavity
- Detach the syringe and fill it with air. Reattach it and express the material aspirated onto a glass slide
- Smear the material and rapidly air dry
Testes
Where testicular atrophy is a possibility, biopsy is preferred to FNA. Semen evaluation may also provide information about testicular lesions, although this is usually used to evaluate sperm quality and quantity.
Indications
Unilateral or bilateral enlargement of the testes
Contraindications
- Bleeding disorders
- Some authors suggest that aspiration of the epididymis is generally contraindicated due to the risk of granuloma formation
Equipment
- 25g, 1-1½” needle, with a 5-10ml syringe attached
- Clean glass slides
Method
- Prepare the skin as for any aseptic surgical procedure
- Use local anaesthesia of the skin to prevent discomfort and to make it easier to direct the needle
- Gently aspirate the testes while moving the needle within the tissue. Testicular cells may be very fragile and it may help to use a non-aspirational technique in which the needle is redirected within the tissue, without applying any suction
- Smear the material and rapidly air dry. If a large lesion is present several aspirates may provide more information
Please visit www.nwlabs.co.uk or see our current price list for more information