Synovial fluid
Examination of synovial fluid is a powerful diagnostic tool in the investigation of joint disease in dogs, cats and horses.
The level of restraint must be sufficient to allow controlled manipulation of the affected joint. Routine aseptic technique must be used. Manipulation of the joint, particularly where there is joint distension, may allow identification of the joint space.
Sample technique for dogs and cats
In approaching a joint, the flexor surface should be avoided. For most joints, a 2-5ml syringe is used with a 21-23g,⅝-1½” needle. For small joints, for example cat carpus a 25g disposable spinal needle may be used. The needle is advanced gently through the joint capsule, the syringe attached and negative pressure applied. If bone is encountered then the needle should be withdrawn slightly and redirected. The pressure is released before removing the needle from the joint.
Sample technique for horses
Manual restraint or sedation may be required depending upon the individual and routine aseptic technique should be used. A 5ml syringe and a 1”, 18-20g needle is suitable for most joints. Local anaesthesia will assist positioning of the needle. The needle is advanced gently through the joint capsule, the syringe attached and negative pressure applied. If bone is encountered then the needle should be withdrawn slightly and redirected. The pressure is released before removal from the joint. Alternatively, the needle may be inserted and the synovial fluid allowed to drip from the hub into suitable collecting bottles.
Synovial fluid analysis
An assessment of synovial fluid volume and physical appearance should be made during arthrocentesis. The volumes collected from normal canine joints range from 0.01-1.0ml. In horses at least 1ml of fluid can be collected from the major limb joints. Normal synovial fluid is transparent and pale yellow. Discoloration, turbidity and any tendency to clot should be noted.
Normal synovial fluid is thixotropic and may become gelatinous on standing, changes reverse when shaken. Processing depends upon the volume of fluid obtained. Samples should be prioritised as follows:
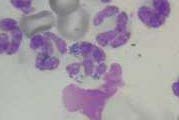
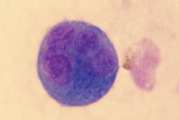
Direct smears. Direct smears should be made immediately by expelling a small amount of synovial fluid onto clean glass slides. The drop can be spread as for a blood smear (Fig 1) but for more viscous samples, squash smears are preferred (Fig 2). Rapidly air dry the smears and do not fix or cover-slip.
Bacterial cultures. Where the differential diagnosis includes septic arthritis, samples should be taken for bacterial culture, preferably before antibiotic therapy is started. This laboratory recommends the inoculation of synovial fluid into a bottle of culture medium (supplied free of charge). First remove the foil cover and swab the bung with alcohol. Replace the needle used for arthrocentesis for a sterile one and transfer 0.5ml of synovial fluid to the culture. Do not remove the cap from the blood culture bottle. Please include in the history any treatment the patient has received in the previous 2-3 days, particularly antibiotics.
Samples in anticoagulant. EDTA is recommended for cell counts, protein concentrations, viscosity assessment and sediment smears. A plain sample is required for the mucin clot. Dispense 0.5-2ml of synovial fluid into EDTA, decant any remaining fluid into a plain tube.