Difference between revisions of "Strongyles - Donkey"
(No difference)
| |
Latest revision as of 12:03, 21 November 2016
Introduction
Small strongyles – Cyathostomins
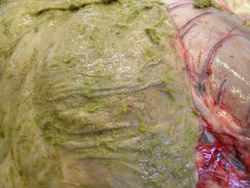

Cyathostomins were formerly called trichonemes or cyathostomes. Adult worms live in the large intestine and have a non-migratory life cycle. Infections with these nematodes typically involve very large populations and numerous species. Over 50 species of cyathostomins have been recorded in horses, donkeys and zebras (Lichtenfels et al, 1998). The species of cyathostomins described in donkeys are similar to those described in horses, but ten species were found to be specific or more prevalent in donkeys and zebras.
After a marked decrease in the prevalence of the large strongyle S. vulgaris following the release of macrocyclic lactones at the end of the last century, there has been an increasing number of reports of serious intestinal diseases attributed to cyathostomins in horses. They are now considered one of the major causes of colic in equines in most regions of the developed world.
The main features of this group are as follows:
- Adult cyathostomins produce typical strongyle eggs indistinguishable from large strongyle eggs
- Eggs excreted in faeces hatch in favourable conditions to third stage larvae (L3)
- L3 larvae are resistant to desiccation and harsh climatic conditions
- Ingested L3 larvae do not penetrate deep tissues but undergo development in the tissue of the large intestine
- The larvae are able to go into a dormant or hypobiotic state and encyst in the epithelial cells of the large intestine
- The encysted larvae are fairly resistant to anthelmintics including ivermectin. Moxidectin (Fort Dodge claims that 80% of encysted developing larvae of small strongyles are eliminated) is effective against some of the developing encysted larvae
- The mechanism that triggers the larvae to enter or come out of hypobiosis has not yet been fully investigated, but may be associated with the elimination of adults in the gut following anthelmintic treatment
Acute verminous enteritis (larval cyathostomosis)
This occurs in late winter or early spring as larvae emerge in large numbers simultaneously otherwise known as ‘mass emergence’.
Diagnosis
History
- Known to be grazing contaminated land
- Previous high egg counts or lack of egg output followed by ‘spikes’ in output
- No known parasitology history
- High stocking density
- Poor pasture hygiene
Clinical signs
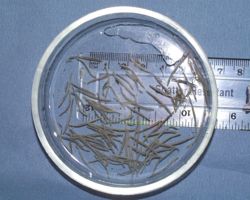
There is often an absence of clinical signs accompanying low-medium grade cyathostomosis in donkeys and mules, the mechanisms for which are not fully understood. Concurrent illness or donkeys suffering immuno-compromisation may become symptomatic in which case the following clinical signs may be seen:
- Digestive upset/colic
- Ill thrift
- Weight loss
Acute verminous enteritis (larval cyathostomosis or mass emergence)
- Initially symptoms of abdominal pain, anorexia and depression are presented and hyperlipaemia quickly follows
- Severe inflammation with sub-mucosal oedema and haemorrhage in the caecum and colon may result in severe diarrhoea but, in the donkey, death due to ‘toxic shock’ may occur before diarrhoea develops
A second form of this larval cyathostomosis has been reported in the horse:
- Diarrhoea is not a feature
- Signs of ill thrift and weight loss are seen
- It occurs in the grazing season
- The progressive accumulation of hypobiotic larvae causes thickened intestinal mucosa
- Thickened mucosa leads to poor intestinal absorption of nutrients
Laboratory tests
- Faecal examination for eggs or larvae
Identification of eggs in the faeces does not indicate the presence of a clinical disease, and only when large numbers of eggs per gram (epg) have been identified can the possibility of clinical disease be considered. Conversely, as the disease may be caused by the larval stages of the parasites, then the epg may be within normal limits whilst resultant disease is present.
- Haematology: red blood cell count, serum protein level
Both forms of acute verminous enteritis (larval cyathostomosis) may be difficult to diagnose ante-mortem from faeces, as the donkeys affected are often on anthelmintics, which will control egg-shedding by adult worms. The parasite may also still be in its prepatent period. At post-mortem the mucosa of the caecum and colon is congested with gross oedema of the sub-mucosa. Ruptured cysts are numerous.
Treatment
Anthelmintics should not be used prophylactically. Blanket treatment with anthelmintics over the last few decades has increased cyathostomin resistance to anthelmintics.
The Donkey Sanctuary uses drugs from all four classes of dewormer to treat clinical cases of suspected cyathostomosis. It is imperative that faecal worm egg count reduction tests are performed on at least a proportion of each group treated to ensure efficacy of treatment. Supportive treatment for hyperlipaemia may also be needed, in the form of prednisolone to suppress an inflammatory response and resulting inappetance.
Elderly and immuno-compromised animals should be treated with caution, ensuring any supportive treatments necessary are given prophylactically prior to or at the time of deworming. Treatment may need to be delayed in extremely unwell animals and should be given at a time when observation post-treatment is possible. The Donkey Sanctuary recommends a different faecal sampling and treatment programme for pregnant jennies and youngstock. More details are available on request.
Control
The anthelmintic control programme operating at The Donkey Sanctuary is reviewed regularly to ensure that:
- it is effective in controlling pasture contamination
- it reduces the incidence of cyathostomosis
- it slows down the development of resistance to anthelmintics
- it is cost-effective. The reduced cost of anthelmintics has offset the increased laboratory costs.
At The Donkey Sanctuary no donkey is given an anthelmintic unless it is deemed necessary by a clinician taking into account the animal’s history and recent FECs. Clinical cases presenting with cyathostomosis are treated with anthelmintics regardless of faecal egg count results. This programme means regularly examining the faeces of all the donkeys, as opposed to administering treatment to the whole herd. Benefits include:
- To reduce handling and stress to the donkey when the whole herd is dewormed
- To reduce exposure of adult worms to repeated doses of anthelmintic and therefore development of resistance
- To help create a ‘refugia’ of susceptible larvae on the pasture to compete with resistant strains
- To avoid unnecessary environmental pollution with anthelmintics
- To preserve drugs for the future
It is claimed that 99% of worms occur on the pasture, whereas 1% occur in the horse (Rose et al, 2000). This statement is equally applicable to the donkey and so no worm control programme should depend solely on drugs. It should also include good pasture and stable management:
- Good pasture hygiene
- Rotational grazing, proper pasture management and harrowing pastures (when weather conditions are optimal) is most important
- The use of a ‘biological vacuum’ (sheep3 ) is still pertinent to ingest larvae on the pasture and is used on the Donkey Sanctuary’s land both in winter and early spring.
- Removal of dung at least twice a week either manually or mechanically significantly reduces pasture contamination (Corbett et al)
- Faecal sampling should be conducted at least four times a year
- If donkeys are at pasture in the winter months then faecal sampling may need to be carried out throughout the year depending upon weather conditions
- Grazing pasture should be rested for as much of the year as possible
- Managing young stock, which has lower natural immunity to parasites, on separate pastures from older groups
3 The use of sheep may increase the incidence of the stomach worm Trichostrongylus axei, but the pathogenecity of this species in donkeys is rarely a problem.
The above principles can be applied to donkeys in most cases. Owners should always be advised to consult their veterinary surgeon regarding periodicity of faecal sampling/worming with regard to their own specific circumstances.
Under tropical weather conditions in most developing countries, the dry season is not favourable for the development and survival of the free living and parasitic stages and so there is relatively little danger of acquiring infection at this time of year. Generally, animals have a high probability of acquiring infection from pasture during and immediately following wet seasons. Treating donkeys at the beginning and end of the rainy season therefore seems to be sufficient under such climatic conditions. This is usually practised in Ethiopia and other developing countries where the farmers cannot afford frequent treatment for their animals.
The efficacy of worming strategies in any country should be carefully monitored by laboratory analysis and condition scoring. An epidemiological assessment of weight loss or anaemia should complement any monitoring process to determine the impact of dental disease and/or poor nutrition.
Literature Search
Use these links to find recent scientific publications via CAB Abstracts (log in required unless accessing from a subscribing organisation).
Strongyles in donkeys publications
References
- Christopher J Corbett, Sandy Love, Anna Moore, Faith A. Burden, Jacqui. B. Matthews, Matthew Denwood. January 2014. The effectiveness of faecal removal methods of pasture management to control the cyathostomin burden of donkeys. Parasites and Vectors. 7:48.
- Lichtenfels, J.R., Kharchenko, V.A., Krecek, R.C., and Gibbons, L.M. (1998). ‘An annotated checklist by genus and species of 93 species level names for 51 recognised species of small strongyles (Nematoda: Strongyloidea: Cyathostominea) of horses, asses and zebras of the world’. Vet. Parasitol., 79. pp 65-79.
- Pandey, V.S., Eysker, M. (1989). ‘Strongylus vulgaris in donkeys (Equus asinus) from the highveld of Zimbabwe’. Vet. Parasitol'. 32. pp 173-179.
- Rose, R.J., Hodgson, D.R. (2000). Manual of Equine Practice. W.B Saunders, Pennsylvania, USA.
- Trawford, A.F., Morriss, C.J., Bell, N.J., and Reid, S.W.J. (2001). ‘Comparing the Efficacy of Moxidectin with Ivermectin in the Donkey’. Paper presented at: The 18th International Conference of the World Association for the Advancement of Veterinary Parasitology. 26-30 August. Stressa, Italy.
- Trawford, A. and Getachew, M. (2008) Parasites In Svendsen, E.D., Duncan, J. and Hadrill, D. (2008) The Professional Handbook of the Donkey, 4th edition, Whittet Books, Chapter 6
|
|
This section was sponsored and content provided by THE DONKEY SANCTUARY |
|---|
