|
|
| (9 intermediate revisions by 2 users not shown) |
| Line 9: |
Line 9: |
| | | | |
| | | | |
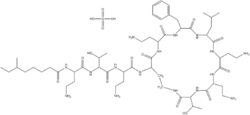
| − | [[Image: Polymyxin B.png|thumb|right|250px|The Core Structure of Polymyxin B (Copyright WikiMedia Commons)]] | + | [[Image: Polymyxin B.png|thumb|right|250px|The Core Structure of Polymyxin B (Cpoyright WikiMedia Commons)]] |
| | | | |
| | This type of antibiotic are basic cyclic decapeptides. The commonly used drugs are '''Polymyxin B and E (Colistin)'''. | | This type of antibiotic are basic cyclic decapeptides. The commonly used drugs are '''Polymyxin B and E (Colistin)'''. |
| Line 15: |
Line 15: |
| | ==Mechanism of Action== | | ==Mechanism of Action== |
| | | | |
| − | This group of antibiotics function by binding to the cell membrane and then making it permeable. This will lead to swelling of the cell and then apoptosis, resulting in the antibiotic being very bactericidal and a concentration dependent killer. As they are cationic basic proteins they also act like detergents.
| |
| | | | |
| − | This group of antibiotics also have another interesting function; they are capable of binding to bacterial endotoxin (LPS) and therefore neutralising it. It is this secondary function, which is often why vets use these antibiotic as they are very useful in treating animals with '''endotoxic shock'''.
| |
| | | | |
| | ==Spectrum of Activity== | | ==Spectrum of Activity== |
| | | | |
| − | Due to nature of their mechanism of action they won't work on gram positive species as they are unable to penetrate the cell wall.
| + | |
| − | * Highly active against gram negatives, including ''Pseudomonas'' species.
| |
| − | * Action is potentiated by cationic detergents (Chlorohexidine) and by chelating agents (EDTA).
| |
| − | * Have a synergistic action with other antibiotics that are able to penetrate the cell wall of gram negatives.
| |
| − | * Their action is inhibited by the presence of cell debris (pus) and divalent cations.
| |
| − | * Able to neutralise bacterial endotoxin.
| |
| | | | |
| | ==Pharmacokinetic Considerations== | | ==Pharmacokinetic Considerations== |
| | | | |
| − | They are strong bases, positively charged at physiological pH and are large molecules. As such they are slowly absorbed by the gastro-intestinal tract and cross into transcellular fluids very poorly. Therefore to provide systemic therapy it must be given parenterally. They bind to cell membranes and tend to accumulate with long term dosing. It is excreted by the urine, totally unchanged, just by glomerular filtration and so have a tendency to accumulate in renal disease.
| |
| | | | |
| | | | |
| | ==Side Effects and Contraindications== | | ==Side Effects and Contraindications== |
| − |
| |
| − | * Colistin is less toxic than Polymyxin B.
| |
| − | * Systemic use can lead to nephrotoxicity, especially in dogs.
| |
| − | * '''Must not use in renal failure patients''' - as will accumulate in the kidney.
| |
| − | * In man it has been known to be neurotoxic and have neuromuscular blocking effects.
| |
| − | * If sulphate salts are used in the drug preperation, pain and necrosis can occur at the injection site.
| |
| − |
| |
| − | {{Learning
| |
| − | |Vetstream = [https://www.vetstream.com/canis/Content/Generic/gen00589.asp Polymyxin B]}}
| |