Difference between revisions of "Toxoplasmosis - Sheep"
| (34 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{unfinished}} |
| − | == | + | |
| − | Toxoplasmosis is the disease caused by '' | + | ==Description== |
| + | |||
| + | Toxoplasmosis is the disease caused by ''Toxoplasma gondii'', an intracelluler protozoan parasite. Although the definitive host is the cat, ''T. gondii'' can infect all mammals including man and is a significant cause of abortion in sheep and goats. Toxoplasmosis does not seem to cause disease in cattle. | ||
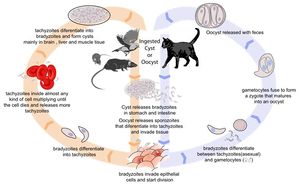
[[Image:Toxoplasmosis Life Cycle.jpg|thumb|right|300px| Life cycle of ''Toxoplasma gondii''. Source: Wikimedia Commons; Author: LadyofHats (2010)]] | [[Image:Toxoplasmosis Life Cycle.jpg|thumb|right|300px| Life cycle of ''Toxoplasma gondii''. Source: Wikimedia Commons; Author: LadyofHats (2010)]] | ||
| + | ===Life Cycle=== | ||
| + | |||
| + | There are three infectious stages of ''Toxoplasma gondii'': 1) sporozoites; 2) actively reproducing tachyzoites; and 3) slowly multiplying bradyzoites. Tachyzoites and bradyzoites are found in tissue cysts, whereas sporozoites are containted within oocysts, which are excreted in the faeces. This means that the protozoa can be transmitted by ingestion of oocyst-contaminated food or water, or by consumption of infected tissue. | ||
| + | |||
| + | In naive cats, ''Toxoplasma gondii'' undergoes an enteroepithelial life cycle. Cats ingests intermediate hosts containing tissue cysts, which release bradyzoites in the gastrointestinal tract. The bradyzoites penetrate the small intestinal epithelium and sexual reproductio ensues, eventually resulting the production of oocysts. Oocysts are passed in the cat's faeces and sporulate to become infectious once in the environment. These can then be ingested by other mammals, including sheep. | ||
| + | |||
| + | When sheep ingest oocysts, ''T.gondii'' intiates extraintestinal replication. This process is the same for all hosts, and also occurs when carnivores ingest tissue cysts in other animals. Sporozoites (or bradyzoites, if cysts are consumed) are released in the intestine to infect the intestinal epithelium where they replicate. This produces tachyzoites, which reproduce asexually within the infected cell. When the infected cell ruptures, tachyzoites are released and disseminate via blood and lymph to infect other tissues. Tachyzoites then replicate intracellularly again and the process continues until the host becomes immune or dies. If the infected cell does not burst, tachyzoites eventually encyst as bradyzoites and persist for the life of the host. Cyst are most commonly found in the brain or skeletal muscle, and are a source of infection for carnivorous hosts. | ||
| + | |||
==Transmission to Sheep== | ==Transmission to Sheep== | ||
| + | |||
===Oocysts in the Environment=== | ===Oocysts in the Environment=== | ||
| − | As the definitive hosts of ''Toxoplasma gondii'', cats become infected when they hunt and eat infected wild rodents and birds. Rodents are a particularly important source of | + | |
| + | As the definitive hosts of ''Toxoplasma gondii'', cats become infected when they hunt and eat infected wild rodents and birds. Rodents are a particularly important source of feline infection, as they can pass ''T. gondii'' infection to their offspring without causing clinical disease. This means that a farm may develop a reservoir of ''T. gondii'' tissue cysts with the potential to cause feline infection and massive oocyst excretion when a cat is introduced to the environment. Between days 3 and 14 post-infection, cats shed over 100 million of oocysts in their faeces. Studies have shown an association between ovine toxoplasma infection, and the contamination of feed or grazing with sporulated oocysts<sup>1</sup>, highligting the importance of oocysts as a source of infection for sheep. It has also been demonstrated that the prevalence of ovine toxoplasmosis varies with the presence of cats on a farm<sup>2</sup>. | ||
===Congenital Transmission=== | ===Congenital Transmission=== | ||
| + | |||
Apart from ingestion of oocysts in the environment, the only other method of transmission of toxoplasmosis to sheep is vertical spread from mother to foetus during pregnancy. This is because sheep are herbivorous, and do not consume animal tissues containing cysts. The outcome of transplacental infection depends on the stage of pregnancy. Infection in early gestation usually causes foetal death, as the foetal immune system is immature at this stage. In mid-gestation, infection may cause the birth of weak or stillborn lambs, sometimes accompanied by a mummified sibling. Ewes infected in the third trimester normally give birth to infected but clinically normal lambs. | Apart from ingestion of oocysts in the environment, the only other method of transmission of toxoplasmosis to sheep is vertical spread from mother to foetus during pregnancy. This is because sheep are herbivorous, and do not consume animal tissues containing cysts. The outcome of transplacental infection depends on the stage of pregnancy. Infection in early gestation usually causes foetal death, as the foetal immune system is immature at this stage. In mid-gestation, infection may cause the birth of weak or stillborn lambs, sometimes accompanied by a mummified sibling. Ewes infected in the third trimester normally give birth to infected but clinically normal lambs. | ||
==Signalment== | ==Signalment== | ||
| − | |||
==Diagnosis== | ==Diagnosis== | ||
| − | |||
===Clinical Signs=== | ===Clinical Signs=== | ||
| − | |||
| − | + | The signs of toxoplasmosis in sheep manifest following the exposure of a naive pregnant ewe to infectious oocysts. This results in primary infection of the ewe and causes transplacental infection of the foetus. Typical clinical signs are therefore abortion and the birth of stillborn or weak lambs. Stillborn or week lambs are often accompanied by a second, mummified foetus. | |
| + | |||
| + | Abortions and neonatal mortality occur when | ||
| + | sheep, (and goats) suffer a primary infection during | ||
| + | pregnancy5. In the UK, toxoplasmosis is a primary | ||
| + | cause of loss in 10-20% of flocks with an abortion | ||
| + | problem, giving an annual incidence in the breeding | ||
| + | ewe population of 1-2%2223. Sporulated T. gondii | ||
| + | oocysts, ingested by susceptible pregnant sheep, | ||
| + | excyst in the digestive tract and release sporozoites | ||
| + | to penetrate the intestinal epithelium. By 4 days, | ||
| + | organisms can be found in the mesenteric lymph | ||
| + | nodes, where they multiply causing marked lymph | ||
| + | node enlargement, sometimes with focal necrosis24. | ||
| + | Around the 5th day toxoplasms are released to cause | ||
| + | a parasitaemia, which may last until the 12th | ||
| + | day25,26. Coinciding with the parasitaemia the ewe | ||
| + | displays a febrile response which can exceed 41°C | ||
| + | around day 6 or 727. | ||
| + | Many tissues become infected in this way. The | ||
| + | cessation of the parasitaemia coincides with the onset | ||
| + | ofan effective maternal immnune response and infection | ||
| + | then persists as bradyzoites within tissue cysts. | ||
| + | In pregnant animals the gravid uterus is an | ||
| + | 'immunologically privileged' site28. On the uterine | ||
| + | side maternal immunological responses are suppreied | ||
| + | while the ability of the fetus, with its placenta, to | ||
| + | recognize and respond to a pathogen commences | ||
| + | during the first half of gestation and develops for the | ||
| + | remainder of pregnancy, so that lambs at birth are | ||
| + | immunocompetent. During a T. gondii parasitaemia | ||
| + | in the dam, tachyzoites are able to parasitise | ||
| + | the caruncular septa, the maternal tissues of the | ||
| + | placentome. They then invade adjacent trophoblast | ||
| + | cells of the fetal villi, and firom there, the rest of the | ||
| + | fetus, between 5 and 10 days after the onset of | ||
| + | parasitaemia9. However, the outcome of infection is | ||
| + | influenced by the stage of gestation at which it | ||
| + | commences. | ||
| + | Infection in early gestation is rapidly fatal18'30 due | ||
| + | to the absence of a fetal immune response to inhibit | ||
| + | parasite multiplication29. Subsequent resorption of | ||
| + | the fetus can be mistaken for infertility30. Infection | ||
| + | in mid gestation may also be fatal and give rise to | ||
| + | a mummified fetus often alongside a sibling which is | ||
| + | born alive but weakly or which dies late in gestation. | ||
| + | Infection in late pregnancy will normally cause fetal | ||
| + | infection but because, at this stage, the competence | ||
| + | of the fetal immune system is well advanced, the | ||
| + | parasite will be resisted and the lamb born live, | ||
| + | infected and immune 8. When infection in the | ||
| + | placentome is initiated, parasite multiplication causes | ||
| + | multiple foci of necrosis29. These foci of tissue | ||
| + | damage expand throughout the remainder of gestation | ||
| + | until abortion or birth when they may be macroscopically | ||
| + | visible as white spots in the cotyledons of | ||
| + | the shed placenta, a feature used to aid diagnozsis"31. | ||
| + | Diagnosis is also helped by histological examination | ||
| + | of the brain where there may be both primary | ||
| + | and secondary lesions32'33. Glial foci, surrounding a | ||
| + | necrotic and sometimes mineralized centre, often | ||
| + | associated with a mild lymphoid meningitis, represent | ||
| + | a fetal immune response following direct damage by | ||
| + | parasite multiplication. Focal leukomalacia is also | ||
| + | common and is thought to be due to fetal anoxia in | ||
| + | late gestation caused by advanced focal necrosis in | ||
| + | the placentome preventing sufficient oxygen transfer | ||
| + | from mother to fetus33. Focal inflammatory lesions | ||
| + | and associated diffuse lymphoid infiltrates may also | ||
| + | be found in the liver, lung and heart and less | ||
| + | frequently in kidneys and skeletal muscle33. | ||
| + | The ovine fetal immune system starts to respond to | ||
| + | T. gondii at or soon after 60 days gestation when both | ||
| + | humoral and cellular reactions can be detected29. | ||
| + | Specific circulating anti-T. gondii IgM and IgG, | ||
| + | detectable after 30 days of maternal infection, can | ||
| + | be used in the diagnosis of T. gondii abortion29. | ||
| + | Infection ofpregnant and non-pregnant ewes provokes | ||
| + | substantial iunity, so that a uterine T. gondii | ||
| + | infection will not develop in a future gestation2l. | ||
===Laboratory Tests=== | ===Laboratory Tests=== | ||
| − | |||
===Pathology=== | ===Pathology=== | ||
| − | |||
| − | + | Aborted ewes show focal necrotic placentitis with white lesions in the cotyledons and foetal tissue | |
| + | |||
| + | Placental | ||
| + | tissue from infected ewes may also show characteristic | ||
| + | gross white spot lesions which are visible to | ||
| + | the naked eye and are areas of necrosis in the tissue | ||
| + | which will limit its effective function in supporting | ||
| + | the pregnancy (Buxton, 1990). | ||
==Treatment== | ==Treatment== | ||
| − | |||
| − | + | *Toxovax vaccine | |
| + | ***Live, avirulent strain of ''Toxoplasma'' | ||
| + | ***Does not form bradyzoites or tissue cysts | ||
| + | ***Killed by host immune system | ||
| + | ***Single dose given 6 weeks before tupping | ||
| + | ***Protects for 2 years | ||
| + | ***Immunity boosted by natural challenge | ||
| + | **Medicated feed can be given daily during the main risk period | ||
| + | ***14 weeks before lambing | ||
| + | **The best method of protection is to prevent cats from contaminating the pasture, lambing sheds and feed stores | ||
| − | The | + | The extent of environmental |
| + | contamination with T. gondii oocysts is thus | ||
| + | related to the distribution and behaviour of cats. | ||
| + | Measures to reduce environmental contamination | ||
| + | by oocysts should be aimed at reducing the number | ||
| + | of cats capable of shedding oocysts. This would include | ||
| + | attempts to limit their breeding. If male cats are | ||
| + | caught, neutered and returned to their colonies the | ||
| + | stability ofthe colony is maintained; fertile male cats | ||
| + | do not challenge the neutered males12 and breeding | ||
| + | is controlled. Thus the maintenance ofa small healthy | ||
| + | population of mature cats will reduce oocyst excretion | ||
| + | as well as help to control rodents. Sheep feed should be | ||
| + | kept covered at all times to prevent its contamination | ||
| + | by cat faeces. | ||
==Prognosis== | ==Prognosis== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Links== | ==Links== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==References== | ==References== | ||
| Line 58: | Line 160: | ||
#Plant, J Wet al (1974) Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. ''Australian Veterinary Journal'', '''50''', 19–21. | #Plant, J Wet al (1974) Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. ''Australian Veterinary Journal'', '''50''', 19–21. | ||
#Skjerve, E et al (1998). Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. ''Preventative Veterinary Medicine'', '''35''', 219–227. | #Skjerve, E et al (1998). Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. ''Preventative Veterinary Medicine'', '''35''', 219–227. | ||
| − | |||
#Buxton, D (1990) Ovine toxoplasmosis: a review. ''Journal of the Royal Society of Medicine'', '''83''', 509-511. | #Buxton, D (1990) Ovine toxoplasmosis: a review. ''Journal of the Royal Society of Medicine'', '''83''', 509-511. | ||
#Innes, E A et al (2009) Ovine toxoplasmosis. ''Parastiology'', '''136''', 1887–1894. | #Innes, E A et al (2009) Ovine toxoplasmosis. ''Parastiology'', '''136''', 1887–1894. | ||
#Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. ''Veterinary Parasitology'', '''147''', 25-28. | #Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. ''Veterinary Parasitology'', '''147''', 25-28. | ||
#Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. ''Veterinary Parasitology'', '''163''', 1-14. | #Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. ''Veterinary Parasitology'', '''163''', 1-14. | ||
| − | + | [[Category:Tissue_Cyst_Forming_Coccidia]][[Category:Sheep]] | |
| − | + | [[Category:To_Do_-_Lizzie]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Category: | ||
| − | [[Category: | ||
Revision as of 15:56, 13 August 2010
| This article is still under construction. |
Description
Toxoplasmosis is the disease caused by Toxoplasma gondii, an intracelluler protozoan parasite. Although the definitive host is the cat, T. gondii can infect all mammals including man and is a significant cause of abortion in sheep and goats. Toxoplasmosis does not seem to cause disease in cattle.
Life Cycle
There are three infectious stages of Toxoplasma gondii: 1) sporozoites; 2) actively reproducing tachyzoites; and 3) slowly multiplying bradyzoites. Tachyzoites and bradyzoites are found in tissue cysts, whereas sporozoites are containted within oocysts, which are excreted in the faeces. This means that the protozoa can be transmitted by ingestion of oocyst-contaminated food or water, or by consumption of infected tissue.
In naive cats, Toxoplasma gondii undergoes an enteroepithelial life cycle. Cats ingests intermediate hosts containing tissue cysts, which release bradyzoites in the gastrointestinal tract. The bradyzoites penetrate the small intestinal epithelium and sexual reproductio ensues, eventually resulting the production of oocysts. Oocysts are passed in the cat's faeces and sporulate to become infectious once in the environment. These can then be ingested by other mammals, including sheep.
When sheep ingest oocysts, T.gondii intiates extraintestinal replication. This process is the same for all hosts, and also occurs when carnivores ingest tissue cysts in other animals. Sporozoites (or bradyzoites, if cysts are consumed) are released in the intestine to infect the intestinal epithelium where they replicate. This produces tachyzoites, which reproduce asexually within the infected cell. When the infected cell ruptures, tachyzoites are released and disseminate via blood and lymph to infect other tissues. Tachyzoites then replicate intracellularly again and the process continues until the host becomes immune or dies. If the infected cell does not burst, tachyzoites eventually encyst as bradyzoites and persist for the life of the host. Cyst are most commonly found in the brain or skeletal muscle, and are a source of infection for carnivorous hosts.
Transmission to Sheep
Oocysts in the Environment
As the definitive hosts of Toxoplasma gondii, cats become infected when they hunt and eat infected wild rodents and birds. Rodents are a particularly important source of feline infection, as they can pass T. gondii infection to their offspring without causing clinical disease. This means that a farm may develop a reservoir of T. gondii tissue cysts with the potential to cause feline infection and massive oocyst excretion when a cat is introduced to the environment. Between days 3 and 14 post-infection, cats shed over 100 million of oocysts in their faeces. Studies have shown an association between ovine toxoplasma infection, and the contamination of feed or grazing with sporulated oocysts1, highligting the importance of oocysts as a source of infection for sheep. It has also been demonstrated that the prevalence of ovine toxoplasmosis varies with the presence of cats on a farm2.
Congenital Transmission
Apart from ingestion of oocysts in the environment, the only other method of transmission of toxoplasmosis to sheep is vertical spread from mother to foetus during pregnancy. This is because sheep are herbivorous, and do not consume animal tissues containing cysts. The outcome of transplacental infection depends on the stage of pregnancy. Infection in early gestation usually causes foetal death, as the foetal immune system is immature at this stage. In mid-gestation, infection may cause the birth of weak or stillborn lambs, sometimes accompanied by a mummified sibling. Ewes infected in the third trimester normally give birth to infected but clinically normal lambs.
Signalment
Diagnosis
Clinical Signs
The signs of toxoplasmosis in sheep manifest following the exposure of a naive pregnant ewe to infectious oocysts. This results in primary infection of the ewe and causes transplacental infection of the foetus. Typical clinical signs are therefore abortion and the birth of stillborn or weak lambs. Stillborn or week lambs are often accompanied by a second, mummified foetus.
Abortions and neonatal mortality occur when sheep, (and goats) suffer a primary infection during pregnancy5. In the UK, toxoplasmosis is a primary cause of loss in 10-20% of flocks with an abortion problem, giving an annual incidence in the breeding ewe population of 1-2%2223. Sporulated T. gondii oocysts, ingested by susceptible pregnant sheep, excyst in the digestive tract and release sporozoites to penetrate the intestinal epithelium. By 4 days, organisms can be found in the mesenteric lymph nodes, where they multiply causing marked lymph node enlargement, sometimes with focal necrosis24. Around the 5th day toxoplasms are released to cause a parasitaemia, which may last until the 12th day25,26. Coinciding with the parasitaemia the ewe displays a febrile response which can exceed 41°C around day 6 or 727. Many tissues become infected in this way. The cessation of the parasitaemia coincides with the onset ofan effective maternal immnune response and infection then persists as bradyzoites within tissue cysts. In pregnant animals the gravid uterus is an 'immunologically privileged' site28. On the uterine side maternal immunological responses are suppreied while the ability of the fetus, with its placenta, to recognize and respond to a pathogen commences during the first half of gestation and develops for the remainder of pregnancy, so that lambs at birth are immunocompetent. During a T. gondii parasitaemia in the dam, tachyzoites are able to parasitise the caruncular septa, the maternal tissues of the placentome. They then invade adjacent trophoblast cells of the fetal villi, and firom there, the rest of the fetus, between 5 and 10 days after the onset of parasitaemia9. However, the outcome of infection is influenced by the stage of gestation at which it commences. Infection in early gestation is rapidly fatal18'30 due to the absence of a fetal immune response to inhibit parasite multiplication29. Subsequent resorption of the fetus can be mistaken for infertility30. Infection in mid gestation may also be fatal and give rise to a mummified fetus often alongside a sibling which is born alive but weakly or which dies late in gestation. Infection in late pregnancy will normally cause fetal infection but because, at this stage, the competence of the fetal immune system is well advanced, the parasite will be resisted and the lamb born live, infected and immune 8. When infection in the placentome is initiated, parasite multiplication causes multiple foci of necrosis29. These foci of tissue damage expand throughout the remainder of gestation until abortion or birth when they may be macroscopically visible as white spots in the cotyledons of the shed placenta, a feature used to aid diagnozsis"31. Diagnosis is also helped by histological examination of the brain where there may be both primary and secondary lesions32'33. Glial foci, surrounding a necrotic and sometimes mineralized centre, often associated with a mild lymphoid meningitis, represent a fetal immune response following direct damage by parasite multiplication. Focal leukomalacia is also common and is thought to be due to fetal anoxia in late gestation caused by advanced focal necrosis in the placentome preventing sufficient oxygen transfer from mother to fetus33. Focal inflammatory lesions and associated diffuse lymphoid infiltrates may also be found in the liver, lung and heart and less frequently in kidneys and skeletal muscle33. The ovine fetal immune system starts to respond to T. gondii at or soon after 60 days gestation when both humoral and cellular reactions can be detected29. Specific circulating anti-T. gondii IgM and IgG, detectable after 30 days of maternal infection, can be used in the diagnosis of T. gondii abortion29. Infection ofpregnant and non-pregnant ewes provokes substantial iunity, so that a uterine T. gondii infection will not develop in a future gestation2l.
Laboratory Tests
Pathology
Aborted ewes show focal necrotic placentitis with white lesions in the cotyledons and foetal tissue
Placental tissue from infected ewes may also show characteristic gross white spot lesions which are visible to the naked eye and are areas of necrosis in the tissue which will limit its effective function in supporting the pregnancy (Buxton, 1990).
Treatment
- Toxovax vaccine
- Live, avirulent strain of Toxoplasma
- Does not form bradyzoites or tissue cysts
- Killed by host immune system
- Single dose given 6 weeks before tupping
- Protects for 2 years
- Immunity boosted by natural challenge
- Medicated feed can be given daily during the main risk period
- 14 weeks before lambing
- The best method of protection is to prevent cats from contaminating the pasture, lambing sheds and feed stores
The extent of environmental contamination with T. gondii oocysts is thus related to the distribution and behaviour of cats. Measures to reduce environmental contamination by oocysts should be aimed at reducing the number of cats capable of shedding oocysts. This would include attempts to limit their breeding. If male cats are caught, neutered and returned to their colonies the stability ofthe colony is maintained; fertile male cats do not challenge the neutered males12 and breeding is controlled. Thus the maintenance ofa small healthy population of mature cats will reduce oocyst excretion as well as help to control rodents. Sheep feed should be kept covered at all times to prevent its contamination by cat faeces.
Prognosis
Links
References
- Plant, J Wet al (1974) Toxoplasma infection and abortion in sheep associated with feeding of grain contaminated with cat faeces. Australian Veterinary Journal, 50, 19–21.
- Skjerve, E et al (1998). Risk factors for the presence of antibodies to Toxoplasma gondii in Norwegian slaughter lambs. Preventative Veterinary Medicine, 35, 219–227.
- Buxton, D (1990) Ovine toxoplasmosis: a review. Journal of the Royal Society of Medicine, 83, 509-511.
- Innes, E A et al (2009) Ovine toxoplasmosis. Parastiology, 136, 1887–1894.
- Buxton, D et all (2007) Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. Veterinary Parasitology, 147, 25-28.
- Dubey, J P (2009) Toxoplasmosis in sheep — The last 20 years. Veterinary Parasitology, 163, 1-14.