Difference between revisions of "Coagulation Tests"
Fiorecastro (talk | contribs) |
m (moved Clotting Factors Tests to Coagulation Tests: Tests are not just for clotting factors) |
||
| (111 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ==Introduction== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | ===Haemostasis=== | ||
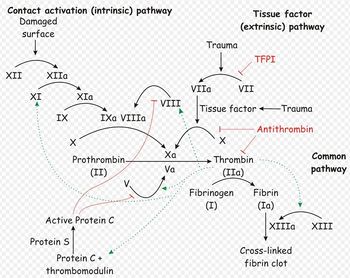
| + | [[Image:Coagulation Cascade.jpg|thumb|right|350px|oagulation cascade. Source: Wikimedia Commons; Author: Joe D (2007)]] | ||
| + | Normally, haemostastis is maintained by three key events. The first stage, primary haemostasis, involves platelets and the blood vessels themselves. It is triggered by injury to a vessel, and platelets become activated, adhere to endothelial connective tissue and aggregate with other platelets. A fragile plug is thus formed which helps to stem haemorrhage from the vessel. Substances are released from platelets during primary haemostasis. Vasoactive compounds give vasoconstriction, and other mediators cause continued platelet activation and aggregation, as well as contraction of the platelet plug. Primary haemostasis ceases once defects in the vessels are sealed and bleeding stops. | ||
| − | + | The platelet plug formed by primary haemostasis is fragile and must be reinforced in order to provide longer-term benefit. In secondary haemostasis, proteinaceous clotting factors interact in a cascade to produce fibrin to reinforce the clot. Two arms of the cascade are activated simultaneously to achieve coagulation: the intrinsic and extrinsic pathways. The intrinsic pathway is activated by contact with collagen due to vessel injury and involves the clotting factors XII, XI, IX and VIII. The extrinsic pathway is triggered by tissue injury and is effected via factor VII. These pathways progress independently before converging at the common pathway, which involves the factors X, V, II and I and ultimately results in the formation of fibrin from fibrinogen. Factors I, VII, IX and X are dependent upon vitamin K to become active. | |
| − | It is therefore important that all aspects of haemostasis can be | + | The end product of haemostasis is a solid clot of fused platelets enclosed in a mesh of fibrin strands. It is important that uncontrolled, widespread clot formation is prevented, and so a fibrinolytic system exists to breakdown fibrin within blood clots. The two most important anticoagulants involved in fibrinolysis are antithrombin III (ATIII) and Protein C. The end products of fibinolysis are fibrin degratation products (FDPs). |
| + | |||
| + | ===Disorders of Haemostasis=== | ||
| + | |||
| + | Abnormalities can develop in any of the components of haemostasis. Disorders of primary haemostasis include vessel defects (i.e. vasculitis), thrombocytopenia (due to decreased production or increased destruction) and abnormalities in platelet function (e.g. congenital defects, disseminated intravascular coagulation). These lead to the occurence of multiple minor bleeds and prolonged bleeding. For example, petechial or ecchymotic haemorrhages may be seen on the skin and mucous membranes, or ocular bleeds may arise. Generally, intact secondary haemostasis prevents major haemorrhage in disorders of primary haemostasis. When secondary haemostasis is abnormal, larger bleeds are frequently seen. Haemothroax, haemoperitoneum, or haemoarthrosis may occur, in addition to subcutaneous and intramuscular haemorrhages. Petechiae and ecchymoses are not usually apparent, as intact primary haemostasis prevents minor capillary bleeding. Examples of secondary haemostatic disorders include clotting factor deficiencies (e.g. hepatic failure, vitamin K deficiency, hereditary disorders) and circulation of substances inhibitory to coagulation (FDPs in disseminated intravascular coagulation, lupus anticoagulant). If fibrinolysis is defective, thrombus formation and infarctions may result. Thrombus formation may be promoted by vascular damage, circulatory stasis or changes in anticoagulants or procoagulants. For example, ATIII may be decreased. This can occur by loss due to glomerular disease or accelarated consumption in disseminated intravascular coagulation or sepsis. | ||
| + | |||
| + | It is therefore important that all aspects of haemostasis can be evaluated. This will help to identify the phase affected and to pinpoint what the abnormality is. There are tests available to assess primary haemostasis, secondary haemostasis and fibrinolysis. | ||
==Tests Evaluating Primary Haemostasis== | ==Tests Evaluating Primary Haemostasis== | ||
| − | Primary haemostasis is dependent on the activity of platelets and, to a lesser extent, the blood vessels themselves. | + | Primary haemostasis is dependent on the activity of platelets and, to a lesser extent, the blood vessels themselves. Platelets are the smallest solid formed component of blood, and are non-nucleated, flattened disc-shaped structures<sup>1</sup>. Their activity leads to vascoconstriction and the formation of platelet plugs to occlude vessel defects. Platelets develop in the bone marrow, and have a life span of around 7.5 days. Around two thirds of platelets are found in the circulation at any one time, with the remainder residing in the spleen<sup>1</sup>. |
| − | Apart from unusual cases involving vasculitis, there are two causes of defects in primary haemostasis: | + | Apart from unusual cases involving vasculitis, there are two causes of defects in primary haemostasis: thrombocytopenia (reduced platelet number), or thrombocytopathia (defective platelet function)<sup>2</sup>. |
===Platelet Number=== | ===Platelet Number=== | ||
| − | |||
| − | The reference range given for platelet number is usually around 200-500x10<sup>9</sup> | + | Platelet numbers may be rapidly estimated by examination of a stained bloof smear, of quatnifies by manual counting techniques or automated haematology analysers. To estimate the platlet number using a blood smear, the average number of platelets in twn oil-immersion fields should be counted and the mean multiplied by 15. |
| + | |||
| + | The reference range given for platelet number is usually around 200-500x10<sup>9</sup>/l, although this varies depending on the laboratory used. Clinical signs due to thrombocytopenia are not commonly encountered until the platelet count drops below 50X10<sup>9</sup>/l, and spontaneous bleeding only occurs with counts lower than 20X10<sup>9</sup>/l. These cut-offs are lowered if platelet function is concurrently affected, for example by the use of non-steroidal anti-inflammatory drugs. | ||
| + | |||
| + | The platelet count is part of a routine coagulation profile or work-up for clotting abnormalities. It is also useful in the assessment of bleeding disorders occurind due to thrombocytopenia, uremia, liver disease or malignancies, and for monitoring the course of disease associated with bone marrow failure<sup>1</sup>. | ||
| + | |||
| + | A platelet count is recommended in the evaluation of all critically ill animals and is vital for patients with bleeding | ||
| + | concerns. A platelet count can be obtained from an automated machine but an estimate of the platelet count can | ||
| + | be made at the bedside from a blood smear. The entire slide should first be scanned for evidence of platelet | ||
| + | clumping that will artificially reduce the platelet count. The number of platelets in several high power fields should | ||
| + | then be counted and an average calculated. There should be 10-15 platelets visible in one high power field (1000 | ||
| + | x magnification with oil immersion). One platelet per high power field represents approximately 15,000 platelets | ||
| + | per microliter. | ||
| + | A normal platelet count is considered to be in the range of 200 – 500,000 and less than 150,000 is generally | ||
| + | considered as thrombocytopenia. Platelet counts less than 50,000 can be associated with increased bleeding | ||
| + | times (see below), platelet counts less than 20,000 may cause clinically concerning bleeding for surgical | ||
| + | procedures and platelet counts less than 5,000 are associated with spontaneous bleeding events. As a guideline | ||
| + | if there are > 5 platelets present per high power field bleeding is most likely not due to thrombocytopenia alone. | ||
| + | Platelet counts are susceptible to falsely low measurements as a result of blood collection and equipment error. | ||
| + | For this reason the diagnosis of thrombocytopenia should always be confirmed prior to initiating major therapy. | ||
| + | |||
| + | ===Buccal Mucosal Bleeding Time=== | ||
| + | The buccal mucosal bleeding time is a simple test that gives a rapid assessment of platelet function, providing platelet numbers are normal. If platelet numbers are below 50x10<sup>9</sup>l, this test should not be performed since bleeding may not be easily stopped. | ||
| − | + | When a small slit is made in the skin, the hemostatic mechanisms necessary for coagulation are activated. Without the aid of external pressure, bleeding usually stops within 7 to 9 minutes. | |
| + | Technique | ||
| − | + | The test is performed using a disposable template that produces a uniform incision. The incision, either horizontal or vertical, is placed on the lateral aspect of the forearm, about 5 cm below the antecubital fossa, after a blood pressure cuff has been inflated to approximately 40 mm Hg. Blood may be absorbed off the skin, but care must be taken to avoid pressure. The time is measured from the moment of incision to the moment bleeding stops. The time may vary based on the commercial template used, the direction of the incision, and the location on the arm. Each institution must establish its own upper limits of normal. | |
| + | Basic Science | ||
| − | + | The vascular platelet phase of hemostasis consists of a primary vasoconstriction that serves to decrease blood flow, followed by adherence of platelets to the ruptured endothelium (adhesion) and each other (aggregation). This platelet aggregate, called the platelet plug, stops the bleeding and forms a matrix for the clot. The bleeding time is an excellent screening test for the vascular platelet phase of hemostasis. It depends on an intact vasospastic response in a small vessel and an adequate number of functionally active platelets. | |
| + | Clinical Significance | ||
| − | + | Patients with abnormalities of the vascular platelet phase of hemostasis present with purpura (petechiae and ecchymoses) and spontaneous bruising. They may have mucosal bleeding and fundus hemorrhages. Commonly, the problem is either thrombocytopenia, easily evaluated by a platelet count, or abnormal platelet function, which can be diagnosed with platelet function studies. The most common acquired platelet function abnormalities are drug induced (aspirin and the nonsteroidal anti-inflammatory agents) and uremia. The most common hereditary abnormality is von Willebrand's disease. | |
==Tests Evaluating Secondary Haemostasis== | ==Tests Evaluating Secondary Haemostasis== | ||
| − | + | ===ACT=== | |
| + | |||
| + | ===PT=== | ||
| + | |||
| + | ===(A)PTT=== | ||
| + | Definition | ||
| + | |||
| + | The aPTT measures the time necessary to generate fibrin from initiation of the intrinsic pathway (Figure 157.1). Activation of factor XII is accomplished with an external agent (e.g., kaolin) capable of activating factor XII without activating factor VII. Since platelet factors are necessary for the cascade to function normally, the test is performed in the presence of a phospholipid emulsion that takes the place of these factors. The classic partial thromboplastin time depends on contact with a glass tube for activation. Since this is considered a difficult variable to control, the "activated" test uses an external source of activation. | ||
| + | Technique | ||
| + | |||
| + | Citrated plasma, an activating agent, and phospholipid are added together and incubated at 37°C. Calcium is added, and the time necessary for the clumping of kaolin is measured. The normal time is usually reported as less than 30 to 35 seconds depending on the technique used. In fact, there is a normal range of about 10 seconds (e.g., 25 to 35), and decreased values ("short") may also be abnormal. | ||
| + | Basic Science | ||
| + | |||
| + | This test is abnormal in the presence of reduced quantities of factors XII, IX, XI, VIII, X, V, prothrombin, and fibrinogen (all integral parts of the "intrinsic" and "common" pathway. It is usually prolonged if a patient has less than approximately 30% normal activity. It can also be abnormal in the presence of a circulating inhibitor to any of the intrinsic pathway factors. The differentiation of inhibitors from factor depletion is important and can best be accomplished by a mixing study in which patient and normal plasma are combined in a 1:1 ratio and the test is repeated on the mixed sample. If the abnormal value is corrected completely, the problem is factor deficiency. If the result does not change or the abnormality is corrected only partially, an inhibitor is present. This difference stems from the above mentioned fact that the aPTT will be normal in the presence of 50% normal activity. | ||
| + | Clinical Significance | ||
| + | |||
| + | The aPTT is a good screening test for inherited or acquired factor deficiencies. Inherited disorders including classic hemophilia A (factor VIII deficiency) and hemophilia B (factor IX deficiency, or Christmas disease) are well-known diseases in which the aPTT is prolonged. Other intrinsic and common pathway factors may also be congenitally absent. These conditions are rare but have been described for all factors. A number of kindreds with abnormalities of factor XII activation have been described. They are usually associated with a prolonged aPTT without clinical signs of bleeding. Acquired factor deficiency is common. Vitamin K deficiency, liver dysfunction, and iatrogenic anticoagulation with warfarin are most common. Factor depletion may also occur in the setting of disseminated intravascular coagulation (DIC), prolonged bleeding, and massive transfusion. | ||
| − | + | A prolonged aPTT that cannot be completely normalized with the addition of normal plasma can be explained only by the presence of a circulating inhibitor of coagulation. The presence of these inhibitors is almost always acquired, and their exact nature is not always apparent. From a clinical point of view, the most common inhibitors should be considered antithrombins. These compounds inhibit the activity of thrombin on the conversion of fibrinogen to fibrin (Figure 157.1). The two most common inhibitors are heparin, which acts through the naturally occurring protein antithrombin III (AT III), and fibrin degradation products (FDP), formed by the action of plasmin on the fibrin clot and usually present in elevated concentrations in DIC and primary fibrinolysis. | |
| − | |||
| − | The | + | Other inhibitors appear to be antibodies. The easiest to understand is the antibody against factor VIII in patients with hemophilia A treated with factor VIII concentrate. Inhibitors against other factors have been described with a variety of diseases that follow a variable course. When characterized, they have been immunoglobulins. |
| − | + | A particular problem may be seen in patients suffering from systemic lupus erythematosus. These patients may present with a prolonged aPTT without evidence of bleeding. Some present with thrombosis. The abnormality cannot be corrected with normal plasma and has been referred to as the "lupus anticoagulant." This phenomenon does not represent an in vivo problem with the coagulation cascade. Rather, it is a laboratory abnormality caused by the presence of a serum constituent that interferes with the in vitro partial thromboplastin test. | |
| − | |||
| − | + | Occasionally the reported value of the aPTT will be lower than normal. This "shortened" time may reflect the presence of increased levels of activated factors in context of a "hypercoagulable state." It is seen in some patients in the early stages of DIC but should not be considered diagnostic for that entity. | |
| − | + | ===PIVKA=== | |
| − | |||
| − | |||
| − | |||
| − | == | + | ==Tests Evaluating Fibrinolysis== |
| − | + | ===Fibrin Degradation Products=== | |
==References== | ==References== | ||
| Line 60: | Line 97: | ||
#Fischbach, F T and Dunning, M B (2008) '''A manual of laboratory and diagnostic tests''', ''Lippincott Williams and Wilkins''. | #Fischbach, F T and Dunning, M B (2008) '''A manual of laboratory and diagnostic tests''', ''Lippincott Williams and Wilkins''. | ||
#Hopper, K (2005) Interpreting coagulation tests. ''Proceedings of the 56th conference of La Società culturale italiana veterinari per animali da compagnia''. | #Hopper, K (2005) Interpreting coagulation tests. ''Proceedings of the 56th conference of La Società culturale italiana veterinari per animali da compagnia''. | ||
| − | |||
| − | |||
#Howard, M R and (2008) '''Haematology: an illustrated colour text''', ''Elsevier Health Sciences''. | #Howard, M R and (2008) '''Haematology: an illustrated colour text''', ''Elsevier Health Sciences''. | ||
| − | # | + | #Walker, K H et al (1990) '''Clinical Methods: The history, Physical and Laboratory Examinations (Third Edition)''', ''Butterworths''. |
| − | + | [[Category:To Do - Blood]][[Category:To Do - Lizzie]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Category:Blood | ||
| − | [[Category: | ||
| − | |||
Revision as of 12:21, 25 August 2010
Introduction
Haemostasis
Normally, haemostastis is maintained by three key events. The first stage, primary haemostasis, involves platelets and the blood vessels themselves. It is triggered by injury to a vessel, and platelets become activated, adhere to endothelial connective tissue and aggregate with other platelets. A fragile plug is thus formed which helps to stem haemorrhage from the vessel. Substances are released from platelets during primary haemostasis. Vasoactive compounds give vasoconstriction, and other mediators cause continued platelet activation and aggregation, as well as contraction of the platelet plug. Primary haemostasis ceases once defects in the vessels are sealed and bleeding stops.
The platelet plug formed by primary haemostasis is fragile and must be reinforced in order to provide longer-term benefit. In secondary haemostasis, proteinaceous clotting factors interact in a cascade to produce fibrin to reinforce the clot. Two arms of the cascade are activated simultaneously to achieve coagulation: the intrinsic and extrinsic pathways. The intrinsic pathway is activated by contact with collagen due to vessel injury and involves the clotting factors XII, XI, IX and VIII. The extrinsic pathway is triggered by tissue injury and is effected via factor VII. These pathways progress independently before converging at the common pathway, which involves the factors X, V, II and I and ultimately results in the formation of fibrin from fibrinogen. Factors I, VII, IX and X are dependent upon vitamin K to become active.
The end product of haemostasis is a solid clot of fused platelets enclosed in a mesh of fibrin strands. It is important that uncontrolled, widespread clot formation is prevented, and so a fibrinolytic system exists to breakdown fibrin within blood clots. The two most important anticoagulants involved in fibrinolysis are antithrombin III (ATIII) and Protein C. The end products of fibinolysis are fibrin degratation products (FDPs).
Disorders of Haemostasis
Abnormalities can develop in any of the components of haemostasis. Disorders of primary haemostasis include vessel defects (i.e. vasculitis), thrombocytopenia (due to decreased production or increased destruction) and abnormalities in platelet function (e.g. congenital defects, disseminated intravascular coagulation). These lead to the occurence of multiple minor bleeds and prolonged bleeding. For example, petechial or ecchymotic haemorrhages may be seen on the skin and mucous membranes, or ocular bleeds may arise. Generally, intact secondary haemostasis prevents major haemorrhage in disorders of primary haemostasis. When secondary haemostasis is abnormal, larger bleeds are frequently seen. Haemothroax, haemoperitoneum, or haemoarthrosis may occur, in addition to subcutaneous and intramuscular haemorrhages. Petechiae and ecchymoses are not usually apparent, as intact primary haemostasis prevents minor capillary bleeding. Examples of secondary haemostatic disorders include clotting factor deficiencies (e.g. hepatic failure, vitamin K deficiency, hereditary disorders) and circulation of substances inhibitory to coagulation (FDPs in disseminated intravascular coagulation, lupus anticoagulant). If fibrinolysis is defective, thrombus formation and infarctions may result. Thrombus formation may be promoted by vascular damage, circulatory stasis or changes in anticoagulants or procoagulants. For example, ATIII may be decreased. This can occur by loss due to glomerular disease or accelarated consumption in disseminated intravascular coagulation or sepsis.
It is therefore important that all aspects of haemostasis can be evaluated. This will help to identify the phase affected and to pinpoint what the abnormality is. There are tests available to assess primary haemostasis, secondary haemostasis and fibrinolysis.
Tests Evaluating Primary Haemostasis
Primary haemostasis is dependent on the activity of platelets and, to a lesser extent, the blood vessels themselves. Platelets are the smallest solid formed component of blood, and are non-nucleated, flattened disc-shaped structures1. Their activity leads to vascoconstriction and the formation of platelet plugs to occlude vessel defects. Platelets develop in the bone marrow, and have a life span of around 7.5 days. Around two thirds of platelets are found in the circulation at any one time, with the remainder residing in the spleen1.
Apart from unusual cases involving vasculitis, there are two causes of defects in primary haemostasis: thrombocytopenia (reduced platelet number), or thrombocytopathia (defective platelet function)2.
Platelet Number
Platelet numbers may be rapidly estimated by examination of a stained bloof smear, of quatnifies by manual counting techniques or automated haematology analysers. To estimate the platlet number using a blood smear, the average number of platelets in twn oil-immersion fields should be counted and the mean multiplied by 15.
The reference range given for platelet number is usually around 200-500x109/l, although this varies depending on the laboratory used. Clinical signs due to thrombocytopenia are not commonly encountered until the platelet count drops below 50X109/l, and spontaneous bleeding only occurs with counts lower than 20X109/l. These cut-offs are lowered if platelet function is concurrently affected, for example by the use of non-steroidal anti-inflammatory drugs.
The platelet count is part of a routine coagulation profile or work-up for clotting abnormalities. It is also useful in the assessment of bleeding disorders occurind due to thrombocytopenia, uremia, liver disease or malignancies, and for monitoring the course of disease associated with bone marrow failure1.
A platelet count is recommended in the evaluation of all critically ill animals and is vital for patients with bleeding concerns. A platelet count can be obtained from an automated machine but an estimate of the platelet count can be made at the bedside from a blood smear. The entire slide should first be scanned for evidence of platelet clumping that will artificially reduce the platelet count. The number of platelets in several high power fields should then be counted and an average calculated. There should be 10-15 platelets visible in one high power field (1000 x magnification with oil immersion). One platelet per high power field represents approximately 15,000 platelets per microliter. A normal platelet count is considered to be in the range of 200 – 500,000 and less than 150,000 is generally considered as thrombocytopenia. Platelet counts less than 50,000 can be associated with increased bleeding times (see below), platelet counts less than 20,000 may cause clinically concerning bleeding for surgical procedures and platelet counts less than 5,000 are associated with spontaneous bleeding events. As a guideline if there are > 5 platelets present per high power field bleeding is most likely not due to thrombocytopenia alone. Platelet counts are susceptible to falsely low measurements as a result of blood collection and equipment error. For this reason the diagnosis of thrombocytopenia should always be confirmed prior to initiating major therapy.
Buccal Mucosal Bleeding Time
The buccal mucosal bleeding time is a simple test that gives a rapid assessment of platelet function, providing platelet numbers are normal. If platelet numbers are below 50x109l, this test should not be performed since bleeding may not be easily stopped.
When a small slit is made in the skin, the hemostatic mechanisms necessary for coagulation are activated. Without the aid of external pressure, bleeding usually stops within 7 to 9 minutes. Technique
The test is performed using a disposable template that produces a uniform incision. The incision, either horizontal or vertical, is placed on the lateral aspect of the forearm, about 5 cm below the antecubital fossa, after a blood pressure cuff has been inflated to approximately 40 mm Hg. Blood may be absorbed off the skin, but care must be taken to avoid pressure. The time is measured from the moment of incision to the moment bleeding stops. The time may vary based on the commercial template used, the direction of the incision, and the location on the arm. Each institution must establish its own upper limits of normal. Basic Science
The vascular platelet phase of hemostasis consists of a primary vasoconstriction that serves to decrease blood flow, followed by adherence of platelets to the ruptured endothelium (adhesion) and each other (aggregation). This platelet aggregate, called the platelet plug, stops the bleeding and forms a matrix for the clot. The bleeding time is an excellent screening test for the vascular platelet phase of hemostasis. It depends on an intact vasospastic response in a small vessel and an adequate number of functionally active platelets. Clinical Significance
Patients with abnormalities of the vascular platelet phase of hemostasis present with purpura (petechiae and ecchymoses) and spontaneous bruising. They may have mucosal bleeding and fundus hemorrhages. Commonly, the problem is either thrombocytopenia, easily evaluated by a platelet count, or abnormal platelet function, which can be diagnosed with platelet function studies. The most common acquired platelet function abnormalities are drug induced (aspirin and the nonsteroidal anti-inflammatory agents) and uremia. The most common hereditary abnormality is von Willebrand's disease.
Tests Evaluating Secondary Haemostasis
ACT
PT
(A)PTT
Definition
The aPTT measures the time necessary to generate fibrin from initiation of the intrinsic pathway (Figure 157.1). Activation of factor XII is accomplished with an external agent (e.g., kaolin) capable of activating factor XII without activating factor VII. Since platelet factors are necessary for the cascade to function normally, the test is performed in the presence of a phospholipid emulsion that takes the place of these factors. The classic partial thromboplastin time depends on contact with a glass tube for activation. Since this is considered a difficult variable to control, the "activated" test uses an external source of activation. Technique
Citrated plasma, an activating agent, and phospholipid are added together and incubated at 37°C. Calcium is added, and the time necessary for the clumping of kaolin is measured. The normal time is usually reported as less than 30 to 35 seconds depending on the technique used. In fact, there is a normal range of about 10 seconds (e.g., 25 to 35), and decreased values ("short") may also be abnormal. Basic Science
This test is abnormal in the presence of reduced quantities of factors XII, IX, XI, VIII, X, V, prothrombin, and fibrinogen (all integral parts of the "intrinsic" and "common" pathway. It is usually prolonged if a patient has less than approximately 30% normal activity. It can also be abnormal in the presence of a circulating inhibitor to any of the intrinsic pathway factors. The differentiation of inhibitors from factor depletion is important and can best be accomplished by a mixing study in which patient and normal plasma are combined in a 1:1 ratio and the test is repeated on the mixed sample. If the abnormal value is corrected completely, the problem is factor deficiency. If the result does not change or the abnormality is corrected only partially, an inhibitor is present. This difference stems from the above mentioned fact that the aPTT will be normal in the presence of 50% normal activity. Clinical Significance
The aPTT is a good screening test for inherited or acquired factor deficiencies. Inherited disorders including classic hemophilia A (factor VIII deficiency) and hemophilia B (factor IX deficiency, or Christmas disease) are well-known diseases in which the aPTT is prolonged. Other intrinsic and common pathway factors may also be congenitally absent. These conditions are rare but have been described for all factors. A number of kindreds with abnormalities of factor XII activation have been described. They are usually associated with a prolonged aPTT without clinical signs of bleeding. Acquired factor deficiency is common. Vitamin K deficiency, liver dysfunction, and iatrogenic anticoagulation with warfarin are most common. Factor depletion may also occur in the setting of disseminated intravascular coagulation (DIC), prolonged bleeding, and massive transfusion.
A prolonged aPTT that cannot be completely normalized with the addition of normal plasma can be explained only by the presence of a circulating inhibitor of coagulation. The presence of these inhibitors is almost always acquired, and their exact nature is not always apparent. From a clinical point of view, the most common inhibitors should be considered antithrombins. These compounds inhibit the activity of thrombin on the conversion of fibrinogen to fibrin (Figure 157.1). The two most common inhibitors are heparin, which acts through the naturally occurring protein antithrombin III (AT III), and fibrin degradation products (FDP), formed by the action of plasmin on the fibrin clot and usually present in elevated concentrations in DIC and primary fibrinolysis.
Other inhibitors appear to be antibodies. The easiest to understand is the antibody against factor VIII in patients with hemophilia A treated with factor VIII concentrate. Inhibitors against other factors have been described with a variety of diseases that follow a variable course. When characterized, they have been immunoglobulins.
A particular problem may be seen in patients suffering from systemic lupus erythematosus. These patients may present with a prolonged aPTT without evidence of bleeding. Some present with thrombosis. The abnormality cannot be corrected with normal plasma and has been referred to as the "lupus anticoagulant." This phenomenon does not represent an in vivo problem with the coagulation cascade. Rather, it is a laboratory abnormality caused by the presence of a serum constituent that interferes with the in vitro partial thromboplastin test.
Occasionally the reported value of the aPTT will be lower than normal. This "shortened" time may reflect the presence of increased levels of activated factors in context of a "hypercoagulable state." It is seen in some patients in the early stages of DIC but should not be considered diagnostic for that entity.
PIVKA
Tests Evaluating Fibrinolysis
Fibrin Degradation Products
References
- Fischbach, F T and Dunning, M B (2008) A manual of laboratory and diagnostic tests, Lippincott Williams and Wilkins.
- Hopper, K (2005) Interpreting coagulation tests. Proceedings of the 56th conference of La Società culturale italiana veterinari per animali da compagnia.
- Howard, M R and (2008) Haematology: an illustrated colour text, Elsevier Health Sciences.
- Walker, K H et al (1990) Clinical Methods: The history, Physical and Laboratory Examinations (Third Edition), Butterworths.