|
|
| (66 intermediate revisions by 3 users not shown) |
| Line 1: |
Line 1: |
| − | {{OpenPagesTop}} | + | {{review}} |
| − | Also known as: '''''MVD — Mitral Valve Disease — Mitral Insufficiency — Mitral Endocardiosis — Myxomatous Mitral Valve Disease (MMVD) — Endocardiosis — Mitral Regurgitation — Chronic Valvular Disease'''''
| |
| | | | |
| − | == Introduction ==
| + | Also known as: '''''MVD — Mitral Valve Disease — Mitral insufficiency — Mitral endocardiosis — Myxomatous Mitral Valve Disease (MMVD) - Endocardiosis''''' |
| − | Myxomatous degeneration of the mitral valve is the most common acquired cardiac disease in the dog. Degenerative mitral valve disease (DMVD) is a progressive disease and subtle changes in valve structure precede the development of clinically significant disease. The aetiology of DMVD is unknown. Genetic predisposition for development of the disease is likely, however the inheritance is complex. | |
| | | | |
| | + | ==Introduction== |
| | + | [[Image:AV valve dysplasia cat.jpg|right|thumb|200px|<small><center>'''Mitral dyplasia'''. Courtesy of A. Jefferies</center></small>]] |
| | + | Mitral valve dysplasia is a congenital malformation or degeneration of the mitral valve leaflets and its supporting structures (''chordae tendinae'', papillary muscles, valvular leaflets, annulus) resulting in valvular regurgitation (insufficiency). It is common in dogs and cats and rare in other species. |
| | | | |
| − | The '''mitral apparatus''' consists of the mitral '''valve leaflets''', valve '''annulus''', '''chordae tendinae''' and '''papillary muscles'''. The mitral valve leaflets are known as '''anterior''' and '''posterior''' leaflets. In the normal dog, these are thin, translucent structures that are anchored to the papillary muscles by chordae tendinae. Both papillary muscles (anterior and posterior) arise from the left ventricular free wall. The mitral valve prevents the backflow of blood from the left ventricle to the left atrium during systole. In early systole, when left ventricular pressure exceeds left atrial pressure, the mitral valve leaflets close. In normal dogs, the chordae tendinae tether the leaflets to prevent them prolapsing into the left atrium. When the mitral valve is incompetent, there is ''regurgitation'' of blood from the left ventricle to the left atrium. Mitral regurgitation may be mild, with no clinical consequence, or may be severe. The severity of mitral regurgitation is determined primarily by the size of the orifice, that results from incomplete apposition of the mitral valve leaflets, and the relationship between left ventricular and left atrial systolic pressures. Mitral regurgitation causes an increase in left atrial pressure, which over time can lead to left atrial dilation. In diastole, the left ventricle is filled by both pulmonary venous return and blood that has been regurgitated into the left atrium. Therefore, both the left atrium and left ventricle become volume overloaded. This may result in '''ventricular dilation and eccentric hypertrophy'''. In severe cases, increased left ventricular and left atrial filling pressures may result. This leads to an increase in pulmonary venous pressure and may result in [[Heart Failure, Left-Sided|left-sided congestive heart failure]].
| + | Chronic mitral regurgitation leads to volume overload of the left heart, which results in dilatation (eccentric hypertrophy) of the left ventricle and atrium. When mitral regurgitation is severe, cardiac output decreases, which results in signs of left sided cardiac failure (LCHF) and pulmonary venous congestion. Dilatation of the left-sided chambers predisposes affected animals to [[:Category:Arrhythmia|arrhythmias]]. In some cases, malformation of the mitral valve complex causes a degree of valvular stenosis as well as insufficiency. |
| | + | |
| | + | In advanced cases, signs of right sided congestive heart failure may follow due to an increased pressure load on the right ventricle as a result of long standing pulmonary congestion. |
| | | | |
| | ==Signalment== | | ==Signalment== |
| | + | Typically in middle aged to older small breed dogs. Genetically predisposed breeds include Cavalier King Charles Spaniel, Bull Terriers, German Shepherds, and Great Danes. |
| | | | |
| − | Degenerative mitral valve disease tends to affect middle-aged and older dogs, particularly males. The disease more commonly affects small breed dogs, with Cavalier King Charles Spaniels, Chihuahuas, Boston Terriers, Poodles, Pomeranians and Bull Terriers being predisposed. The disease is also recognized in large breed dogs.
| + | ==Diagnosis== |
| | + | ===History=== |
| | + | Animals may remain asymptomatic for years. Typical reported signs include exercise intolerance and dyspnoea as a result of reduced cardiac output and a ventilation perfusion mismatch due to pulmonary oedema. A progressive cough often during rest or recumbency is frequently seen and needs to be distinguished from primary respiratory disease. Sudden death is possible due to a left atrial tear or advanced pulmonary oedema. |
| | | | |
| − | ==History and Clinical Signs== | + | ===Clinical Signs=== |
| − | Animals may remain asymptomatic for years, the disease is usually clinically silent until it is advanced.
| + | * Left apical systolic murmur |
| | + | * Left sided congestive heart failure |
| | + | **Resting Tachycardia |
| | + | **Pale Mucous membranes |
| | + | ** Prolonged Capillary refill time (CRT) |
| | + | ** Prolonged Jugular filling time |
| | + | ** Pulmonary crackles / evidence of pulmonary oedema |
| | + | ** Cool extremities |
| | + | ** Loss of sinus arrhythmia |
| | + | ** Cardiac arrhythmias e.g. Atrial fibrillation, Atrial premature complexes |
| | | | |
| − | In most affected dogs, DMVD does not cause clinical signs and the disease is detected by the auscultation of a cardiac murmur at routine health checks.
| + | ===Diagnostic imaging=== |
| − | | + | ====Radiography==== |
| − | In cases where DMVD becomes clinically significant, a '''cough''' is usually the first clinical sign noticed by the owner. The coughing is likely of multifactorial aetiology and may be related to pulmonary oedema, stimulation of the juxtapulmonary (J) receptors that are associated with pulmonary capillaries and detect increases in pulmonary venous pressure, compression of a mainstem bronchi by an enlarged left atrium and concurrent airway disease. Occasionally, '''syncope''' is the first sign of clinically significant DMVD. This may occur due to arrhythmias or on exertion where mitral regurgitation limits stroke volume and therefore cardiac output.
| + | Left lateral, right lateral and ventrodorsal views of the thorax are needed. The key radiographic signs associated with mitral valve dysplasia and resulting left sided congestive heart failure are cardiomegaly, pulmonary venous congestion (enlarged pulmonary arteries and veins) and pulmonary oedema. Cardiomegaly may lead to dorsal displacement of the trachea. |
| − | | |
| − | Other symptoms that may occur include increased exercise intolerance and efficiency, tachypnea or dyspnea during exercise, ascites, weight loss, anorexia and thromboembolisms.
| |
| − | | |
| − | == Diagnosis== | |
| − | ===Physical Examination=== | |
| − | * Systolic murmur with point of maximal intensity over the left apex. Murmur grade is usually correlated with severity of mitral regurgitation, severe regurgitation causes a loud murmur.
| |
| − | * Mid-systolic click, associated with mitral prolapse. In many dogs, clicks are a precursor to mitral regurgitation.
| |
| − | | |
| − | Other findings will depend on the stage of disease. Crackles may be detected on thoracic auscultation in patients with pulmonary oedema, resulting from [[Heart Failure, Left-Sided|left-sided congestive heart failure]]. Abdominal palpation is usually normal, but ascites and hepatomegaly may be present when there is concurrent [[Heart Failure, Right-Sided|right-sided congestive heart failure]].
| |
| | | | |
| − | Primary respiratory disease, such as chronic bronchitis, is also common in older small breed dogs. It is important to distinguish between the patient with clinically significant respiratory disease and incidental DMVD from the patient with clinically significant DMVD. Respiratory sinus arrhythmia, indicating vagal influence on heart rate and rhythm, is usually not present in severe cardiac disease. In contrast, sinus arrhythmia is usually preserved or accentuated when respiratory disease is the cause of clinical signs.
| + | Evidence of right sided congestive heart failure maybe evident in severe cases e.g. distended caudal vena cava, hepatomegaly, ascites, pleural effusions. |
| − | | |
| − | ===Diagnostic Imaging===
| |
| − | ====Radiography====
| |
| − | Early in the course of DMVD, thoracic radiographs will be normal. As the disease progresses, cardiomegaly will become apparent. There may be evidence of left atrial enlargement, with or without dorsal displacement of the trachea and narrowing of the mainstem bronchus. Pulmonary venous distension may be observed if there is increased pulmonary venous pressure. Interstitial pulmonary oedema may precede alveolar pulmonary oedema. Evidence of [[Heart Failure, Right-Sided|right-sided congestive heart failure]] may be present in severe cases, radiographic findings include distension of the caudal vena cava, hepatomegaly, ascites and pleural effusion.
| |
| | | | |
| | ====Echocardiography==== | | ====Echocardiography==== |
| − | * Thickened mitral valve leaflets
| + | Evidence of left atrial and left ventricular enlargement is visible on echocardiography. The 'fractional shortening' is also increased which is measured as the percentage change in the left ventricular diameter during systole and is used as a measure of systolic function. It is also possible to see structural changes in the valve leaflets in some cases. The regurgitant jet of blood can be detected using colour doppler and evidence of turbulent flow. |
| − | * Prolapse of mitral valve leaflets into the left atrium during systole
| |
| − | * Tricuspid leaflets may also be affected, though usually not as severely as the mitral valve
| |
| − | * Increased diastolic left ventricular diameter
| |
| − | * Hyperdynamic left ventricle
| |
| − | * Colour Doppler jet of mitral regurgitation
| |
| − | *(Flail leaflet)
| |
| − | | |
| − | Thickening of the mitral valve leaflets is usually diffuse, but most pronounced at the leaflet edges. With myxomatous degeneration, the mitral valve becomes stiffer and distorted. The conformation of the valve remains constant throughout the cardiac cycle. Normally, the mitral valve leaflets do not extend beyond a line across the mitral annulus in systole. In dogs with DMVD, the mitral leaflets prolapse towards the left atrium during systole. Colour Doppler can be used to demonstrate the jet of mitral regurgitation. The size of the jet is related to the severity of mitral regurgitation. Most mitral regurgitation jets in DMVD are eccentric.
| |
| − | | |
| − | The more severe the DMVD, the greater the degree of left ventricular and left atrial dilation. | |
| − | | |
| − | Fractional shortening may be increased (hyperynamic left ventricle). This is because, in the setting of mitral regurgitation, impedance to ventricular emptying is reduced (blood can be ejected into the low pressure left atrium)and end-diastolic ventricular stretch is increased by the addition of the regurgitant fraction, increasing the force of contraction.
| |
| − | | |
| − | A serious complication of DMVD is '''chordae tendinae rupture''', resulting in a 'flail leaflet' and acute worsening of mitral regurgitation. A leaflet segment typically 'flails' back into the left atrium during systole.
| |
| − | | |
| − | '''Left atrial rupture''' is a major complication of DMVD. Left atrial endocardial and endomyocardial splits are usually multiple and may heal or perforate the atrial wall, causing haemopericardium or an acquired atrial septal defect depending on their depth and location.
| |
| | | | |
| | ===Electrocardiogram (ECG)=== | | ===Electrocardiogram (ECG)=== |
| − | Electrocardiography is primarily used to diagnose arrhythmias, but can provide evidence of chamber enlargement. Most arrhythmias in DMVD are supraventricular in origin and occur secondary to left atrial stretch. Ventricular arrhythmias may develop in association with left ventricular dilation and fibrosis.
| + | A resting ECG trace may show evidence of an enlarge left atrium (wide P wave), an enlarged left ventricle (tall R wave, wide QRS complex, shift of mean electrical axis to the left) and rhythm disturbances such as sinus tachycardia, atrial fibrillation, atrial premature complexes and atrial tachycardia. |
| − | | |
| − | * P-mitrale: wide P waves in leads II, III and aVF, indicates left atrial enlargement
| |
| − | * high R-wave
| |
| − | * Stage C2-D: (supra-) ventricular extrasystoles
| |
| | | | |
| | ===Laboratory Tests=== | | ===Laboratory Tests=== |
| − | Pro-brain natriuretic peptide ('''NT-proBNP''') concentration is associated with severity of DMVD. Elevated NT-proBNP levels are useful in discriminating patients with respiratory distress caused by heart failure from those with primary respiratory tract disease. | + | Pro-brain natriuretic peptide (N-BNP) is a newly described cardiac hormone considered to be an effective marker of severity and prognosis of acute coronary syndromes and congestive heart failure. Circulating levels of the hormone increase in peripheral blood with increased myocardial stress. Commercial assays are not currently available. |
| − | | |
| − | ==Staging==
| |
| − | Staging according to American College of Veterinary Internal Medicine (ACVIM) is as follows:
| |
| − | * '''Stage A''': Dog predisposed to the development of DMVD
| |
| − | * '''Stage B''': Subclinical disease
| |
| − | ** ''B1'': Without cardiac remodeling
| |
| − | ** ''B2'': With cardiac remodeling
| |
| − | * '''Stage C''': Current or prior clinical signs
| |
| − | * '''Stage D''': Refractory heart failure
| |
| − | | |
| − | == Treatment ==
| |
| − | ===Stage B===
| |
| − | There is no therapy demonstrated to be beneficial in dogs with stage B1 disease. Nevertheless the cardiac function should be controlled after 12 months or earlier if the general condition gets worse.
| |
| − | | |
| − | The results of the EPIC study demonstrated that administration of '''Pimobendan''' to dogs with stage B2 disease resulted in prolongation of the asymptomatic phase of disease by approximately 15 months. Dogs receiving Pimobendan were around 33% less likely to go into congestive heart failure or suffer a cardiac death than those not receiving the drug. Pimobendan appears safe and well-tolerated. The cardiac function should be controlled after 6-12 months.
| |
| − | | |
| − | Based on findings of the EPIC study, dogs with typical mitral valve murmurs of grade III/VI or higher should be investigated to look for evidence of cardiomegaly. If cardiomegaly is apparent, then the dog may benefit from starting Pimobendan, as opposed to the 'watch and wait' approach that was previously recommended.
| |
| − | | |
| − | ===Stage C===
| |
| − | Medical management is intended to alleviate clinical signs and prolong life.
| |
| − | | |
| − | '''Furosemide''' is a potent first-line diuretic that can be administered orally or parenterally, depending on the clinical status of the patient. Most patients with congestive heart failure secondary to DMVD require lifelong diuretic therapy.
| |
| − | | |
| − | The addition of an '''ACE inhibitor''' is considered standard therapy. The benefits of ACE inhibitors are related to their vasodilator action and also protecting the heart from the detrimental effects of RAAS activation.
| |
| − | | |
| − | '''Pimobendan''' is phosphodiesterase inhibitor and calcium sensitiser that is both a ''positive inotrope'' and ''vasodilator'' (inodilator). A randomized clinical trial (QUEST) demonstrated a survival benefit associated with Pimobendan administration, when evaluated relative to treatment which was considered at that time to be the gold standard; benazepril. Use of triple therapy with furosemide, an ACE inhibitor and Pimobendan is recommended. When financial or compliance concerns limit the therapeutic choices, evidence suggests that Pimobendan is superior to an ACE inhibitor.
| |
| − | | |
| − | Aldosterone may contribute to the development of myocardial fibrosis. Complete suppression of RAAS is generally not achieved by ACE inhibition alone. Therefore the addition of '''Spironolactone''' may be beneficial.
| |
| − | | |
| − | Surgical mitral valve repair in dogs is currently being performed. However, availability is limited by the expense, required expertise and cardiopulmonary bypass facilities.
| |
| − | | |
| − | If the patient shows supraventricular tachycardia '''Digoxin''' can be prescribed. If that does not work effectively '''calcium canal blockers''' can be added.
| |
| − | | |
| − | Relevant ventricular extrasystoles can be treated with '''sodium canal blockers.'''
| |
| − | | |
| − | Syncopes or acsites can be treated with '''Sildenafil.'''
| |
| − | | |
| − | '''Amlodipin''' can be used to treat systemic hypertension that occurs as a result to the valvular degeneration.
| |
| − | | |
| − | | |
| − | ''<u>Clinical Example:</u>''
| |
| − | | |
| − | A patient is presented in an acute cardiac crisis. He is staged as C3-D. The therapy goal is to get him to C1.
| |
| − | | |
| − | # inpatient admission
| |
| − | # '''Furosemide'''
| |
| − | #* depending on the degree of severity: 2-4 mg/kg body weight, parenteral, every 2-6 hours
| |
| − | #* better is an intravenous drip that covers half of the conservation needs (1 mg/kg body weight per hour), can be reduced by half when the breathing rate has normalized
| |
| − | # '''Pimobendan'''
| |
| − | #* 0,15 mg/kg body weight once i.v.;
| |
| − | #* when the symptoms persist the injection can be repeated after 12 hours
| |
| − | # Oxygen
| |
| − | # thoracocentesis if there is a liquidothorax
| |
| − | # punction of the ascites if there is a right heart failure
| |
| − | # '''Dobutamin''': 5–10µg/kg body weight/min (Stadium D)
| |
| − | # '''Nitroglycerin''' with an atomizer: 1–2 pumps in the mouth (cave: do not breath in, wear gloves!)
| |
| − | # '''ACE inhibitors''' and '''Spironolactone''' should be added as soon as oral treatment is possible
| |
| | | | |
| | + | ==Treatment== |
| | + | No treatment is recommended prior to the onset of heart failure. Once there is evidence of congestive heart failure, treatment is aimed at its management through a combination of drugs. |
| | | | |
| − | '''''When the patient is stabilized:'''''
| + | The aims of treatment are to: |
| | | | |
| − | # treatment can be continued at home
| + | 1. '''Reduce Preload''' |
| − | # '''Sildenafil''' for the pulmonary hypertension
| + | ::Diuretics to reduce circulating fluid volume (Frusemide, Benzofluazide, Spironolactone, Amiloride) |
| | + | ::Vasodilators to reduce venous return (Nitrates, ACE inhibitors, Alpha antagonists) |
| | + | 2. '''Reduce Afterload |
| | + | ::Vasodilators to decrease systemic vascular resistance |
| | + | :::ACE inhibitors e.g. Enalapril, Benzapril, Imidopril |
| | + | :::Pimobendan |
| | + | :::Calcium channel blockers e.g. Amlodipine |
| | + | :::Nitrates e.g. Nitroprusside |
| | + | 3. '''Enhance Systolic function |
| | + | ::Positive inotropes to increase cardiac contractility and increase cardiac output (Pimobendan, Digoxin, Dobutamine, Xanthines) |
| | + | 4. '''Improve Diastolic function |
| | + | ::Negative chronotropes to increase the length of diastole (Digoxin, Atenolol) |
| | + | ::Calcium channel blockers to improve relaxation (Amlodipine) |
| | + | 5. '''Control cardiac arrhythmias using anti-arrhythmic drugs |
| | | | |
| − | ===Stage D=== | + | ==Prognosis== |
| − | If congestive heart failure signs are not controlled by high doses of Furosemide, addition of a thiazide and Spironolactone should be considered. Together these drugs have a synergistic action, by providing sequential nephron blockade, allowing lower doses of the individual agents.
| + | Mitral Valve Dysplasia can remain asymptomatic for many years (average 4 years). Once congestive heart failure has developed, the progression of the diseae can be monitored by the severity of the clinical signs (cough, exercise intolerance) and radiographically looking at cardiac size, the degree of pulmonary oedema and the size of the left atrium. Cardiac size can be measured objectively using the Vertebral Heart Score method. |
| − | | + | Mean survival is 200-300 days once in overt cardiac failure with standard treatment protocols. |
| − | ==Monitoring and Follow Up==
| |
| − | For dogs in stage B, owners should be made aware of signs of congestive heart failure. In dogs with Stage B2 disease where congestive heart failure is imminent, it is useful to give the owner Furosemide to administer if the dog develops signs of respiratory distress. Owners of Stage B2 and Stage C dogs should be educated on how to measure sleeping respiratory rate and should begin recording this regularly.
| |
| − | | |
| − | The frequency of follow-up examinations is dependent on the severity of disease and owner compliance. For dogs with preclinical disease, rechecks can be recommended every 6-12 months depending on the severity of mitral regurgitation and cardiac remodeling. Following hospitalization for control of acute congestive heart failure, dogs should receive a follow up examination within 2 weeks to check for resolution of clinical signs, hydration status, electrolytes and renal function. For dogs in stage C with stable disease, re-checks can be every 3-6 months.
| |
| − | | |
| − | == Prognosis ==
| |
| − | | |
| − | Asymptomatic patients may live for many years. Dogs with stage B2 disease have a median of 27 months before developing congestive heart failure. Once heart failure occurs, life expectancy is usually around 6-12 months, although some patients remain stable for longer. Risk factors for progression include severity of valvular lesions, increased age and male gender. Risk factors for onset of congestive heart failure include severity of mitral regurgitation, left atrial enlargement and elevated NT-proBNP. Development of complications such as atrial fibrillation or chordae tendinae rupture are associated with a poor prognosis.
| |
| − | | |
| − | | |
| − | {{Learning
| |
| − | |videos = [http://www.cardioacademy.cevalearn.com/en/Programme/Sessions/1-Pathophysiology-of-Mitral-Valve-Disease video on mitral valve disease from Cardio Academy]
| |
| − | |flashcards = [[Endocardial Pathology Flashcards]]
| |
| − | |literature search = [http://www.cabdirect.org/search.html?rowId=1&options1=AND&q1=%22Mitral+Valve+Dysplasia%22&occuring1=title&rowId=2&options2=OR&q2=%22Mitral+Valve+Disease%22&occuring2=title&rowId=3&options3=OR&q3=%22Mitral+insufficiency%22&occuring3=title&rowId=4&options4=OR&q4=%22endocardiosis%22&occuring4=title&x=36&y=9&publishedstart=yyyy&publishedend=yyyy&calendarInput=yyyy-mm-dd&la=any&it=any&show=all Mitral Valve Dysplasia publications]
| |
| − | | |
| − | [http://www.cabdirect.org/search.html?it=any&q2=%22Mitral+Valve+Disease%22&q1=%22Mitral+Valve+Dysplasia%22&calendarInput=yyyy-mm-dd&q4=%22endocardiosis%22&q3=%22Mitral+insufficiency%22&occuring1=title&show=all&rowId=1&rowId=2&rowId=3&rowId=4&options1=AND&options2=OR&occuring4=title&options3=OR&options4=OR&occuring3=title&occuring2=title&publishedend=yyyy&la=any&publishedstart=yyyy&fq=sc:(ft+OR+fr+OR+fa+OR+fv+OR+fw+OR+fx+OR+gf+OR+ga+OR+b1+OR+b2+OR+b3+OR+b4+OR+b5+OR+b6)&y=9&x=36 Other MDV Full Text Articles]
| |
| − | |full text = [http://www.cabi.org/cabdirect/FullTextPDF/2010/20103219945.pdf ''' Myxomatous degenerative mitral valve disease: an update.''' Disatian, S.; Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand, Thai Journal of Veterinary Medicine, 2010, 40, 2, pp 151-157, many ref.]
| |
| − | | |
| − | [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093114836.pdf ''' Latest information about canine mitral valve disease: results of the QUEST trial.''' Häggström, J.; The North American Veterinary Conference, Gainesville, USA, Small animal and exotics. Proceedings of the North American Veterinary Conference, Orlando, Florida, USA, 17-21 January, 2009, 2009, pp 188-191, 10 ref. - '''Full Text Article''']
| |
| − | | |
| − | [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093017845.pdf ''' Treatment of mitral valve disease in dogs.''' French, A.; Gething, M.; Jones, B.; Australian Small Animal Veterinary Association, Bondi, Australia, 33rd World Small Animal Veterinary Association Congress, Dublin, Ireland, 20-24 August 2008, 2008, pp 107-108]
| |
| − | | |
| − | [http://www.cabi.org/cabdirect/FullTextPDF/2009/20093017847.pdf ''' Prognostic variables in canine mitral valve disease.''' Häggstrom, J.; Gething, M.; Jones, B.; Australian Small Animal Veterinary Association, Bondi, Australia, 33rd World Small Animal Veterinary Association Congress, Dublin, Ireland, 20-24 August 2008, 2008, pp 112-113, 7 ref.]
| |
| − | }}
| |
| | | | |
| | + | ==Literature Search== |
| | ==References== | | ==References== |
| − | * Tilley,L.P., Smith, F.W.K, Oyama, M., Sleeper, M. (2016) '''Manual of Canine and Feline Cardiology (Fifth Edition)''' ''Saunders''. | + | * Merck & Co (2008) '''The Merck Veterinary Manual (Eighth Edition)''' ''Merial'' |
| − | * Luis Fuentes, V, Johnson, L.R, Dennis, S. (2010) '''BSAVA Manual of Canine and Feline Cardiorespiratory Medicine (Second Edition)''' | + | * Nelson, R.W. and Couto, C.G. (2009) '''Small Animal Internal Medicine (Fourth Edition)''' ''Mosby Elsevier''. |
| − | * Boswood, A. et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study - A Randomized Clinical Trial. JVIM, September 2016. DOI: 10.1111/jvim.14586
| + | * Tilley, L.P. and Smith, F.W.K.(2004)'''The 5-minute Veterinary Consult (Third edition)''' ''Lippincott, Williams & Wilkins''. |
| − | | + | * Tilley,L.P., Smith, F.W.K, Oyama, M., Sleeper, M. (2007) '''Manual of Canine and Feline Cardiology''' ''Saunders''. |
| − | {{review}}
| |
| − | | |
| − | {{OpenPages}}
| |
| | | | |
| − | [[Category:Cardiovascular_System_-_Degenerative_Pathology]] [[Category:Endocardial_Pathology]] [[Category:Expert_Review]] [[Category:Cardiac_Diseases_-_Cat]] [[Category:Cardiac_Diseases_-_Dog]] | + | [[Category:Cardiovascular_System_-_Developmental_Pathology]][[Category:Expert_Review]] |
| − | [[Category:Cardiac_Diseases_-_Horse]]
| |
| − | [[Category:Cardiovascular_System_-_Developmental_Pathology]]
| |
| − | [[Category:Cardiology Section]]
| |
Also known as: MVD — Mitral Valve Disease — Mitral insufficiency — Mitral endocardiosis — Myxomatous Mitral Valve Disease (MMVD) - Endocardiosis
Introduction
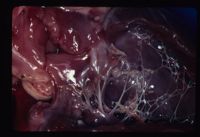
Mitral dyplasia. Courtesy of A. Jefferies Mitral valve dysplasia is a congenital malformation or degeneration of the mitral valve leaflets and its supporting structures (chordae tendinae, papillary muscles, valvular leaflets, annulus) resulting in valvular regurgitation (insufficiency). It is common in dogs and cats and rare in other species.
Chronic mitral regurgitation leads to volume overload of the left heart, which results in dilatation (eccentric hypertrophy) of the left ventricle and atrium. When mitral regurgitation is severe, cardiac output decreases, which results in signs of left sided cardiac failure (LCHF) and pulmonary venous congestion. Dilatation of the left-sided chambers predisposes affected animals to arrhythmias. In some cases, malformation of the mitral valve complex causes a degree of valvular stenosis as well as insufficiency.
In advanced cases, signs of right sided congestive heart failure may follow due to an increased pressure load on the right ventricle as a result of long standing pulmonary congestion.
Signalment
Typically in middle aged to older small breed dogs. Genetically predisposed breeds include Cavalier King Charles Spaniel, Bull Terriers, German Shepherds, and Great Danes.
Diagnosis
History
Animals may remain asymptomatic for years. Typical reported signs include exercise intolerance and dyspnoea as a result of reduced cardiac output and a ventilation perfusion mismatch due to pulmonary oedema. A progressive cough often during rest or recumbency is frequently seen and needs to be distinguished from primary respiratory disease. Sudden death is possible due to a left atrial tear or advanced pulmonary oedema.
Clinical Signs
- Left apical systolic murmur
- Left sided congestive heart failure
- Resting Tachycardia
- Pale Mucous membranes
- Prolonged Capillary refill time (CRT)
- Prolonged Jugular filling time
- Pulmonary crackles / evidence of pulmonary oedema
- Cool extremities
- Loss of sinus arrhythmia
- Cardiac arrhythmias e.g. Atrial fibrillation, Atrial premature complexes
Diagnostic imaging
Radiography
Left lateral, right lateral and ventrodorsal views of the thorax are needed. The key radiographic signs associated with mitral valve dysplasia and resulting left sided congestive heart failure are cardiomegaly, pulmonary venous congestion (enlarged pulmonary arteries and veins) and pulmonary oedema. Cardiomegaly may lead to dorsal displacement of the trachea.
Evidence of right sided congestive heart failure maybe evident in severe cases e.g. distended caudal vena cava, hepatomegaly, ascites, pleural effusions.
Echocardiography
Evidence of left atrial and left ventricular enlargement is visible on echocardiography. The 'fractional shortening' is also increased which is measured as the percentage change in the left ventricular diameter during systole and is used as a measure of systolic function. It is also possible to see structural changes in the valve leaflets in some cases. The regurgitant jet of blood can be detected using colour doppler and evidence of turbulent flow.
Electrocardiogram (ECG)
A resting ECG trace may show evidence of an enlarge left atrium (wide P wave), an enlarged left ventricle (tall R wave, wide QRS complex, shift of mean electrical axis to the left) and rhythm disturbances such as sinus tachycardia, atrial fibrillation, atrial premature complexes and atrial tachycardia.
Laboratory Tests
Pro-brain natriuretic peptide (N-BNP) is a newly described cardiac hormone considered to be an effective marker of severity and prognosis of acute coronary syndromes and congestive heart failure. Circulating levels of the hormone increase in peripheral blood with increased myocardial stress. Commercial assays are not currently available.
Treatment
No treatment is recommended prior to the onset of heart failure. Once there is evidence of congestive heart failure, treatment is aimed at its management through a combination of drugs.
The aims of treatment are to:
1. Reduce Preload
- Diuretics to reduce circulating fluid volume (Frusemide, Benzofluazide, Spironolactone, Amiloride)
- Vasodilators to reduce venous return (Nitrates, ACE inhibitors, Alpha antagonists)
2. Reduce Afterload
- Vasodilators to decrease systemic vascular resistance
- ACE inhibitors e.g. Enalapril, Benzapril, Imidopril
- Pimobendan
- Calcium channel blockers e.g. Amlodipine
- Nitrates e.g. Nitroprusside
3. Enhance Systolic function
- Positive inotropes to increase cardiac contractility and increase cardiac output (Pimobendan, Digoxin, Dobutamine, Xanthines)
4. Improve Diastolic function
- Negative chronotropes to increase the length of diastole (Digoxin, Atenolol)
- Calcium channel blockers to improve relaxation (Amlodipine)
5. Control cardiac arrhythmias using anti-arrhythmic drugs
Prognosis
Mitral Valve Dysplasia can remain asymptomatic for many years (average 4 years). Once congestive heart failure has developed, the progression of the diseae can be monitored by the severity of the clinical signs (cough, exercise intolerance) and radiographically looking at cardiac size, the degree of pulmonary oedema and the size of the left atrium. Cardiac size can be measured objectively using the Vertebral Heart Score method.
Mean survival is 200-300 days once in overt cardiac failure with standard treatment protocols.
Literature Search
References
- Merck & Co (2008) The Merck Veterinary Manual (Eighth Edition) Merial
- Nelson, R.W. and Couto, C.G. (2009) Small Animal Internal Medicine (Fourth Edition) Mosby Elsevier.
- Tilley, L.P. and Smith, F.W.K.(2004)The 5-minute Veterinary Consult (Third edition) Lippincott, Williams & Wilkins.
- Tilley,L.P., Smith, F.W.K, Oyama, M., Sleeper, M. (2007) Manual of Canine and Feline Cardiology Saunders.
