Difference between revisions of "Equine Nervous System - Horse Anatomy"
| (52 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{unfinished}} | {{unfinished}} | ||
==Central Nervous System== | ==Central Nervous System== | ||
| − | ===Brain | + | ===[[Equine Brain - Horse Anatomy|Brain]]=== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | === | + | ===[[Equine Cranial Nerves - Horse Anatomy|Cranial Nerves]]=== |
| − | |||
| − | === | + | ===[[Vasculature of the Equine Brain - Horse Anatomy|Vasculature of the Brain]]=== |
| − | |||
| − | |||
| − | + | ===[[Equine Spinal Cord - Horse Anatomy|Spinal Cord]]=== | |
| − | === | + | ===[[Equine Meninges - Horse Anatomy|Meninges]]=== |
| − | |||
| − | === | + | ===[[Equine Cerebrospinal Fluid - Horse Anatomy|Cerebrospinal Fluid]]=== |
| − | |||
| − | + | ===Clinical Links=== | |
| − | * | + | *[[Equine Protozoal Myeloencephalitis]] |
| − | * | + | *[[Equine Herpesvirus 1]] |
| − | + | *[[Polyneuritis Equi]] | |
| − | The | + | ==Peripheral Nervous System== |
| − | + | ==Introduction== | |
| − | The | + | The '''Peripheral Nervous System''' is made up of cranial and spinal nerves. Spinal nerves are named after the vertebra immediately above it, except for '''cervical vertebra'''. There are '''7''' cervical vertebrae and '''8''' cervical spinal nerves. The peripheral nervous system can be divided into the '''somatic nervous system''' and '''autonomic nervous system'''. The somatic nervous system co-ordinates body movements and also receives external stimuli. It regulates activities that are under conscious control. The autonomic nervous system subdivided into the '''sympathetic nervous system''', '''parasympathetic nervous system''', and enteric division. The sympathetic nervous system is the '''‘fight or flight’''' system which comes into role when an animal is under threat, its main neurotransmitter is '''adrenaline'''. The parasympathetic nervous system is the '''‘rest and digest’''' system which is responsible for digestion. Its main neurotransmitter is '''acetylcholine'''. |
| − | + | ==Structure== | |
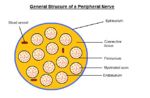
| − | === | + | [[Image:WIKIVETperipheralnervestructure.jpg|thumb|right|150px|Peripheral Nerve Structure. Sophie Stenner, RVC 2008]] |
| − | + | Nerve fibres reside in a connective tissue matrix called the '''endoneurium''' and are gathered together into bundles or fascicles defined by a second connective tissue layer called the '''perineurium'''. Groups of fascicles are then gathered together in a third connective tissue layer called the '''epineurium'''. Thus, peripheral nerves have a '''three-tiered hierarchical arrangement of connective tissue'''. '''Renaut bodies''' are loose, cigar-shaped whorls of extracellular matrix within fascicles that are common in equine nerves at points of stress or compression. | |
| − | + | ==Nerve Fibre== | |
| − | + | The nerve fibre consists of the impulse-carrying axon, which is surrounded by an ensheathing cell, the [[#The Schwann cell|Schwann cell]], which in turn is surrounded by an acellular basal lamina that is continuous along the length of the nerve. Nerve fibres come in various discrete diameter groups, which are reflected in their conduction velocities. The larger the diameter the more rapid the rate of impulse conduction. Particular targets or receptors are associated with axons of a particular diameter. Axons connected to muscles spindles have a large diameter (20 µm) and conduct at 120 m/s whilst the smallest myelinated fibres are about 1µm and conduct at around 6 m/s. The smallest fibres of all are the unmyelinated fibres (the high-threshold sensory afferents, or C-fibres, and post-ganglionic autonomies) and have a diameter of between 1 and 0.1 µm. These fibres do not conduct by saltatory conduction and have very slow conduction rates of around 0.5 m/s. | |
| + | ===Axon=== | ||
| + | Axons have an outer membrane called the '''axolemma''' and within this there is the '''axoplasm''' which is continuous with the cytoplasm of the [[Neurons - Anatomy & Physiology|neuron]]. There are no ribosomes, either free or attached to endoplasmic reticulum in axons and therefore, no protein synthesis. Protein synthesis takes place within the cell body and some dendrites and all protein replacement required for the maintenance of the axon depends on proteins being imported from the cell body. A critical feature of the axon is its '''cytoskeleton''', which consists of two key elements; '''neurofilaments''' and '''microtubules'''. '''Neurofilaments''' are intermediate filaments of about 10 nm diameter, and belong to the same class as other cytoskeletal proteins such as keratin, desmin, vimentin, or GFAP of astrocytes. Neurofilaments are formed from a triplet of polypeptide subunits of heavy (~ 200 kD), medium (~ 150 kD) and low (~ 60 kD) molecular weights. Typically, these subunits are heavily phosphorylated and are more numerous than microtubules, especially in large diameter axons, having a pivotal role in determining axon diameter. They are formed in the cell body, transported down the axon by axoplasmic transport and degraded in the terminals by Ca<sup>2+</sup> activated proteases. In other words, there is a constant turnover of neurofilament within the healthy axon. '''Microtubules''' within axons are similar to microtubules elsewhere, consisting of polymerised dimers of alpha and beta tubulin arranged as a hollow tube of about 28 nm. They are relatively abundant in smaller diameter axons, and are also synthesised in the cell body. An important component of the cytoskeleton are the '''microtubule associated proteins''' or MAP's and the tau proteins. These proteins are important in microtubule assembly and stability. Different classes of MAP's occur in the dendrites and the axons, and to some extent account for the different ultrastructural features that distinguish these two types of neuronal process. They form cross links between adjacent microtubules but also connect to neurofilaments and actin microfilaments, implying complex interactions between the various components of the axon skeleton. | ||
| + | ===Schwann Cell=== | ||
| + | Myelination in the PNS is achieved by the '''Schwann cell''', a derivative of neural crest cells, which bud off from the neuroepithelium at a very early stage of neurogenesis. During development, Schwann cells engage many small axons and as axonal diameter increases, Schwann cells eventually relate with only a single axon c.f [[Neurons - Anatomy & Physiology#Oligodendrocytes|oligodendrocytes]]. This single axon is enveloped in a trough by the Schwann cell processes that engulf it and as the processes come together, an inner '''mesaxon''' is formed. The leading-edge process continues to move over the axon forming a spiral. Myelination, an extremely complex molecular process, occurs when the cytoplasm within the process is extruded allowing the internal surfaces of the membrane to come together as the '''major dense line''', the outer membrane apposition constituting the intraperiod line. The alternating pattern of these two form the lamellae of compacted myelin. The myelin sheath is attached to, and is an integral part of the Schwann cell on which it is dependent for its maintenance. | ||
| − | + | A single Schwann cell forms a single myelin sheath or internode and there is a reasonably constant relationship between the myelin thickness and the internodal length, which in turn is associated with axon calibre. Large axons have long, thick myelin sheaths and therefore conduct more rapidly. The internodes do not abut one another but are separated by an exposed area of axon called the '''node of Ranvier'''. If the axons remain of small diameter, then a Schwann cell will continue to associate with many axons, although none of them are myelinated. Thus, ''even unmyelinated axons retain a Schwann cell ensheathment''. These non-myelinating Schwann cells are sometimes referred to as ''Remak cells.'' | |
| + | ===Axoplasmic Transport=== | ||
| + | Neurons are very large cells and most of a neurons cytoplasm is present in its processes while most of the cells RNA is located in cell body (Nissl substance). These cells have therefore evolved mechanisms to transport large macromolecules and organelles up and down processes. | ||
| + | ===Anterograde Transport=== | ||
| + | Anterograde transport moves substances from the cell body to the axon. Two basic forms of anterograde transport can be recognised: '''fast anterograde transport''' and '''slow anterograde transport'''. Fast anterograde transport allows movement of all membranous organelles such as synaptic vesicles and occurs at a rate of around 400mm/day (recent evidence suggests that there are many forms of fast anterograde transport, mediated by different kinesins). Fast anterograde transport depends critically on oxidative metabolism, and is, in fact independent of the cell body. Microtubules act as a static track along which the organelles can move, driven by the ATPase '''kinesin''' which acts as a "motor" molecule. Fast anterograde transport is independent of the cell body. Anything which interferes with energy supply or cytoskeleton necessary for fast anterograde transport has profound effects on the health of the axon. Agents such as colchicine or vincristine block microtubule assembly, disrupting fast anterograde transport. '''Slow anterograde transport''' deals with cytoskeletal elements and large soluble proteins. Slow anterograde transport can be further sub-divided into a slow component, which occurs at about 2mm/day (neurofilament, rubulin, actin) and a fast component, which occurs at around 4 mm/day, transporting all other proteins (eg myosin, clathrin). | ||
| + | ===Retrograde Transport=== | ||
| + | Retrograde transport returns materials from the axon terminal to the cell body, either for degradation or restoration and reuse. As with fast anterograde transport, particles move along microtubules. The motor molecule for retrograde transport is '''dynein''' which is a microtubule-associated ATPase. The retrograde transport system is important not only for returning material to the cell body, but also provides the means whereby target-derived trophic factors, such as nerve growth factor (NGF) for dorsal root ganglion neurons, are conveyed to the cell body where they promote cell survival. Research is being undertaken into the use of trophic factors to promote cell survival during degenerative pathology. The retrograde transport system can be "hijacked" by harmful substances to gain entry to the peripheral neuron and ultimately the CNS. [[Herpesviridae|Herpes virus]], [[Tetanus - Horse|tetanus]] and heavy metals all affect the retrograde transport system. | ||
| + | ==Blood Supply== | ||
| + | The epineurium is penetrated by the vascular supply to the nerve and this blood supply is known as the '''vasa nervorum'''. Only capillaries occur within the endoneurial compartment. The capillaries of the endoneurium are joined by tight junctions and provide a barrier to large macromolecules. This forms the basis of the blood-nerve barrier (BNB), which has similarities to the [[Blood Brain Barrier - Anatomy & Physiology|blood-brain barrier]] of the CNS. The BNB appears to be relatively weak in the sensory ganglia because fenestrations occur between endothelial cells in this location. Sensory ganglia are therefore more vulnerable to blood-borne agents. A further "barrier" is provided by the perineurium which consists of sheets of flattened cells, connected by tight junctions and covered on both sides by a basal lamina. The only route across this structure is trans- rather than inter-cellular. | ||
| − | + | [[Category:To Do - AP Review]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 10:46, 22 November 2012
| This article is still under construction. |
Central Nervous System
Brain
Cranial Nerves
Vasculature of the Brain
Spinal Cord
Meninges
Cerebrospinal Fluid
Clinical Links
Peripheral Nervous System
Introduction
The Peripheral Nervous System is made up of cranial and spinal nerves. Spinal nerves are named after the vertebra immediately above it, except for cervical vertebra. There are 7 cervical vertebrae and 8 cervical spinal nerves. The peripheral nervous system can be divided into the somatic nervous system and autonomic nervous system. The somatic nervous system co-ordinates body movements and also receives external stimuli. It regulates activities that are under conscious control. The autonomic nervous system subdivided into the sympathetic nervous system, parasympathetic nervous system, and enteric division. The sympathetic nervous system is the ‘fight or flight’ system which comes into role when an animal is under threat, its main neurotransmitter is adrenaline. The parasympathetic nervous system is the ‘rest and digest’ system which is responsible for digestion. Its main neurotransmitter is acetylcholine.
Structure
Nerve fibres reside in a connective tissue matrix called the endoneurium and are gathered together into bundles or fascicles defined by a second connective tissue layer called the perineurium. Groups of fascicles are then gathered together in a third connective tissue layer called the epineurium. Thus, peripheral nerves have a three-tiered hierarchical arrangement of connective tissue. Renaut bodies are loose, cigar-shaped whorls of extracellular matrix within fascicles that are common in equine nerves at points of stress or compression.
Nerve Fibre
The nerve fibre consists of the impulse-carrying axon, which is surrounded by an ensheathing cell, the Schwann cell, which in turn is surrounded by an acellular basal lamina that is continuous along the length of the nerve. Nerve fibres come in various discrete diameter groups, which are reflected in their conduction velocities. The larger the diameter the more rapid the rate of impulse conduction. Particular targets or receptors are associated with axons of a particular diameter. Axons connected to muscles spindles have a large diameter (20 µm) and conduct at 120 m/s whilst the smallest myelinated fibres are about 1µm and conduct at around 6 m/s. The smallest fibres of all are the unmyelinated fibres (the high-threshold sensory afferents, or C-fibres, and post-ganglionic autonomies) and have a diameter of between 1 and 0.1 µm. These fibres do not conduct by saltatory conduction and have very slow conduction rates of around 0.5 m/s.
Axon
Axons have an outer membrane called the axolemma and within this there is the axoplasm which is continuous with the cytoplasm of the neuron. There are no ribosomes, either free or attached to endoplasmic reticulum in axons and therefore, no protein synthesis. Protein synthesis takes place within the cell body and some dendrites and all protein replacement required for the maintenance of the axon depends on proteins being imported from the cell body. A critical feature of the axon is its cytoskeleton, which consists of two key elements; neurofilaments and microtubules. Neurofilaments are intermediate filaments of about 10 nm diameter, and belong to the same class as other cytoskeletal proteins such as keratin, desmin, vimentin, or GFAP of astrocytes. Neurofilaments are formed from a triplet of polypeptide subunits of heavy (~ 200 kD), medium (~ 150 kD) and low (~ 60 kD) molecular weights. Typically, these subunits are heavily phosphorylated and are more numerous than microtubules, especially in large diameter axons, having a pivotal role in determining axon diameter. They are formed in the cell body, transported down the axon by axoplasmic transport and degraded in the terminals by Ca2+ activated proteases. In other words, there is a constant turnover of neurofilament within the healthy axon. Microtubules within axons are similar to microtubules elsewhere, consisting of polymerised dimers of alpha and beta tubulin arranged as a hollow tube of about 28 nm. They are relatively abundant in smaller diameter axons, and are also synthesised in the cell body. An important component of the cytoskeleton are the microtubule associated proteins or MAP's and the tau proteins. These proteins are important in microtubule assembly and stability. Different classes of MAP's occur in the dendrites and the axons, and to some extent account for the different ultrastructural features that distinguish these two types of neuronal process. They form cross links between adjacent microtubules but also connect to neurofilaments and actin microfilaments, implying complex interactions between the various components of the axon skeleton.
Schwann Cell
Myelination in the PNS is achieved by the Schwann cell, a derivative of neural crest cells, which bud off from the neuroepithelium at a very early stage of neurogenesis. During development, Schwann cells engage many small axons and as axonal diameter increases, Schwann cells eventually relate with only a single axon c.f oligodendrocytes. This single axon is enveloped in a trough by the Schwann cell processes that engulf it and as the processes come together, an inner mesaxon is formed. The leading-edge process continues to move over the axon forming a spiral. Myelination, an extremely complex molecular process, occurs when the cytoplasm within the process is extruded allowing the internal surfaces of the membrane to come together as the major dense line, the outer membrane apposition constituting the intraperiod line. The alternating pattern of these two form the lamellae of compacted myelin. The myelin sheath is attached to, and is an integral part of the Schwann cell on which it is dependent for its maintenance.
A single Schwann cell forms a single myelin sheath or internode and there is a reasonably constant relationship between the myelin thickness and the internodal length, which in turn is associated with axon calibre. Large axons have long, thick myelin sheaths and therefore conduct more rapidly. The internodes do not abut one another but are separated by an exposed area of axon called the node of Ranvier. If the axons remain of small diameter, then a Schwann cell will continue to associate with many axons, although none of them are myelinated. Thus, even unmyelinated axons retain a Schwann cell ensheathment. These non-myelinating Schwann cells are sometimes referred to as Remak cells.
Axoplasmic Transport
Neurons are very large cells and most of a neurons cytoplasm is present in its processes while most of the cells RNA is located in cell body (Nissl substance). These cells have therefore evolved mechanisms to transport large macromolecules and organelles up and down processes.
Anterograde Transport
Anterograde transport moves substances from the cell body to the axon. Two basic forms of anterograde transport can be recognised: fast anterograde transport and slow anterograde transport. Fast anterograde transport allows movement of all membranous organelles such as synaptic vesicles and occurs at a rate of around 400mm/day (recent evidence suggests that there are many forms of fast anterograde transport, mediated by different kinesins). Fast anterograde transport depends critically on oxidative metabolism, and is, in fact independent of the cell body. Microtubules act as a static track along which the organelles can move, driven by the ATPase kinesin which acts as a "motor" molecule. Fast anterograde transport is independent of the cell body. Anything which interferes with energy supply or cytoskeleton necessary for fast anterograde transport has profound effects on the health of the axon. Agents such as colchicine or vincristine block microtubule assembly, disrupting fast anterograde transport. Slow anterograde transport deals with cytoskeletal elements and large soluble proteins. Slow anterograde transport can be further sub-divided into a slow component, which occurs at about 2mm/day (neurofilament, rubulin, actin) and a fast component, which occurs at around 4 mm/day, transporting all other proteins (eg myosin, clathrin).
Retrograde Transport
Retrograde transport returns materials from the axon terminal to the cell body, either for degradation or restoration and reuse. As with fast anterograde transport, particles move along microtubules. The motor molecule for retrograde transport is dynein which is a microtubule-associated ATPase. The retrograde transport system is important not only for returning material to the cell body, but also provides the means whereby target-derived trophic factors, such as nerve growth factor (NGF) for dorsal root ganglion neurons, are conveyed to the cell body where they promote cell survival. Research is being undertaken into the use of trophic factors to promote cell survival during degenerative pathology. The retrograde transport system can be "hijacked" by harmful substances to gain entry to the peripheral neuron and ultimately the CNS. Herpes virus, tetanus and heavy metals all affect the retrograde transport system.
Blood Supply
The epineurium is penetrated by the vascular supply to the nerve and this blood supply is known as the vasa nervorum. Only capillaries occur within the endoneurial compartment. The capillaries of the endoneurium are joined by tight junctions and provide a barrier to large macromolecules. This forms the basis of the blood-nerve barrier (BNB), which has similarities to the blood-brain barrier of the CNS. The BNB appears to be relatively weak in the sensory ganglia because fenestrations occur between endothelial cells in this location. Sensory ganglia are therefore more vulnerable to blood-borne agents. A further "barrier" is provided by the perineurium which consists of sheets of flattened cells, connected by tight junctions and covered on both sides by a basal lamina. The only route across this structure is trans- rather than inter-cellular.