Difference between revisions of "Immunofluorescence"
| (10 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | Also known as: '''''Fluorescent Antibody Test — FAT''''' | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Introduction== | ==Introduction== | ||
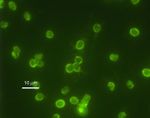
| − | [[Image:763px-Cryptosporidium parvum 01.jpg|thumb|right|150px|Immunofluorescence of ''Cryptosporidium parvum'' spores]] | + | [[Image:763px-Cryptosporidium parvum 01.jpg|thumb|right|150px|Immunofluorescence of ''Cryptosporidium parvum'' spores, EPA/H.D.A. Lindquist, '''WikiMedia Commons''', 2006]] |
| − | Immunofluorescence is a technique used to detect cell or tissue-associated antigens using antibodies | + | Immunofluorescence is a technique used to detect cell or tissue-associated antigens using antibodies labeled with fluorescent tags. The stained tissues are then detected by immunofluorescence microscopy (qualitative) or flow cytometry (quantitative). Antibodies bind stably and specifically to their corresponding antigen and the technique makes use of the fact that they can be coupled to fluorescent dyes, such as fluorescein and rhodamine, with no effect on specificity. These conjugates bind to antigens present in a sample and can then be visualised under a microscope with a suitable light source, such as UV light. |
==Fluorescent dyes== | ==Fluorescent dyes== | ||
| − | + | If a molecule has the property of fluorescence, it can absorb light of one wavelength (excitation) and emit light of another (emission). Antibodies tagged with these dyes (known as '''fluorochromes''') form immune complexes with specific antigens which can then be indirectly visualised when excited by light of the appropriate wavelength. | |
| − | If a molecule has the property of fluorescence, it can absorb light of one wavelength ( | ||
===Commonly used fluorochromes=== | ===Commonly used fluorochromes=== | ||
| Line 26: | Line 18: | ||
*An antibody directed against a specific antigen is directly conjugated with the fluorescent dye and applied to the sample. | *An antibody directed against a specific antigen is directly conjugated with the fluorescent dye and applied to the sample. | ||
'''Indirect staining''' | '''Indirect staining''' | ||
| − | *Utilizes a double layer technique- a primary, unlabelled antibody is applied to the sample, followed by a secondary antibody, an anti-immunoglobulin that has been conjugated to a fluorochrome. | + | *Utilizes a double layer technique - a primary, unlabelled antibody is applied to the sample, followed by a secondary antibody, an anti-immunoglobulin that has been conjugated to a fluorochrome. |
**Indirect staining has several advantages: | **Indirect staining has several advantages: | ||
***Several secondary antibodies bind to each primary antibody, so the resulting fluorescence is brighter than that of the direct staining. | ***Several secondary antibodies bind to each primary antibody, so the resulting fluorescence is brighter than that of the direct staining. | ||
***One preparation of secondary antibody can be used to test many sera | ***One preparation of secondary antibody can be used to test many sera | ||
***By using a mixture of primary antibodies, it is possible to detect the relative expressions of different antigens in the same cell | ***By using a mixture of primary antibodies, it is possible to detect the relative expressions of different antigens in the same cell | ||
| − | ***Quite often loss of antibody is sustained during the conjugation- in the indirect method the primary antibodies do not need to be conjugated, so this limiting factor is reduced. | + | ***Quite often loss of antibody is sustained during the conjugation- in the indirect method the primary antibodies do not need to be conjugated, so this limiting factor is reduced. |
==Applications== | ==Applications== | ||
| Line 53: | Line 45: | ||
*The flow cytometer is an instrument that directs cells in single file through a narrow chamber illuminated by a laser beam. As fluorescently-tagged cells pass through the laser beam, the fluorochrome is excited and emits light that is detected by a PMT-like detector | *The flow cytometer is an instrument that directs cells in single file through a narrow chamber illuminated by a laser beam. As fluorescently-tagged cells pass through the laser beam, the fluorochrome is excited and emits light that is detected by a PMT-like detector | ||
*This detector is capable of measuring the strength of emission, so it is possible to sort cells that are negative, slightly positive and highly positive for a specific antigen | *This detector is capable of measuring the strength of emission, so it is possible to sort cells that are negative, slightly positive and highly positive for a specific antigen | ||
| − | * | + | *Flow cytometry provides a rapid quantitative technique for antigen detection |
| + | |||
| + | |||
| + | {{review}} | ||
| + | <br><br> | ||
| + | {{Jim Bee 2007}} | ||
| + | [[Category:Immunological Testing]] | ||
Latest revision as of 16:51, 17 March 2012
Also known as: Fluorescent Antibody Test — FAT
Introduction
Immunofluorescence is a technique used to detect cell or tissue-associated antigens using antibodies labeled with fluorescent tags. The stained tissues are then detected by immunofluorescence microscopy (qualitative) or flow cytometry (quantitative). Antibodies bind stably and specifically to their corresponding antigen and the technique makes use of the fact that they can be coupled to fluorescent dyes, such as fluorescein and rhodamine, with no effect on specificity. These conjugates bind to antigens present in a sample and can then be visualised under a microscope with a suitable light source, such as UV light.
Fluorescent dyes
If a molecule has the property of fluorescence, it can absorb light of one wavelength (excitation) and emit light of another (emission). Antibodies tagged with these dyes (known as fluorochromes) form immune complexes with specific antigens which can then be indirectly visualised when excited by light of the appropriate wavelength.
Commonly used fluorochromes
- Fluorescein: organic (carbon-based) dye, most widely used. Absorbs blue (490nm) and emits yellow green fluorescence (517nm)
- Rhodamine: organic dye, absorbs yellow-green light and emits deep red fluorescence (546nm).
- As fluorescein and rhodamine fluorescences are easy to distinguish from one another, it is possible to conjugate them to different antibodies and simultaneously visualise two different antigens on the same cell or tissue.
- Phycoerythrin: can absorb light from the blue-green (495nm) and the yellow wavelengths, emits bright red fluorescence
Techniques
Direct staining
- An antibody directed against a specific antigen is directly conjugated with the fluorescent dye and applied to the sample.
Indirect staining
- Utilizes a double layer technique - a primary, unlabelled antibody is applied to the sample, followed by a secondary antibody, an anti-immunoglobulin that has been conjugated to a fluorochrome.
- Indirect staining has several advantages:
- Several secondary antibodies bind to each primary antibody, so the resulting fluorescence is brighter than that of the direct staining.
- One preparation of secondary antibody can be used to test many sera
- By using a mixture of primary antibodies, it is possible to detect the relative expressions of different antigens in the same cell
- Quite often loss of antibody is sustained during the conjugation- in the indirect method the primary antibodies do not need to be conjugated, so this limiting factor is reduced.
- Indirect staining has several advantages:
Applications
- Immunofluorescence is often used to identify populations of cells, and was successfully employed in the identification of the CD4+ and CD8+ subpopulations of T cells
- Identifying bacterial and viral species
- Detecting antigen-antibody complexes in autoimmune diseases
- Detecting complement components in tissues
- Localising hormones, antigens and other cellular products in tissue samples and subcellular compartments
- Mapping the molecular architecture of tissues in relation to gross anatomy
Developments
Confocal microscopy
- The scanning confocal fluorescent microscope is a recent development that uses computer-aided techniques to produce a high-resolution thin optical section of a sample without the need for further, complex sample preparation. Fluorescent images were previously hard to resolve as the picture was subject to glare from planes above and below that in focus, resulting in a blurred image.
- To obtain an image, a laser light is focused on a fine plane within the sample. The resulting fluorescent emission is collected in a photomultiplier tube (PMT) with a confocal aperture. The fluorescence from planes above and below the optical section fails to reach this aperture, resulting in a sharper image.
- The resolution can be further increased by using low-level illumination, meaning the fluorescent dye can only be excited by two photons. When a pulsed laser is used, it only reaches sufficient intensity to excite fluorescence when the beam is focused onto the focal plane of the microscope. This minimises fluorescence to the desired optical section itself.
Time-lapse video microscopy
- Sensitive digital video cameras can be used to record the movement of fluorescently tagged molecules, for example to track the movement of such molecules in cell membranes or during interaction between cells.
Flow cytometry
- The flow cytometer is an instrument that directs cells in single file through a narrow chamber illuminated by a laser beam. As fluorescently-tagged cells pass through the laser beam, the fluorochrome is excited and emits light that is detected by a PMT-like detector
- This detector is capable of measuring the strength of emission, so it is possible to sort cells that are negative, slightly positive and highly positive for a specific antigen
- Flow cytometry provides a rapid quantitative technique for antigen detection
| This article has been peer reviewed but is awaiting expert review. If you would like to help with this, please see more information about expert reviewing. |
| Originally funded by the RVC Jim Bee Award 2007 |